
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
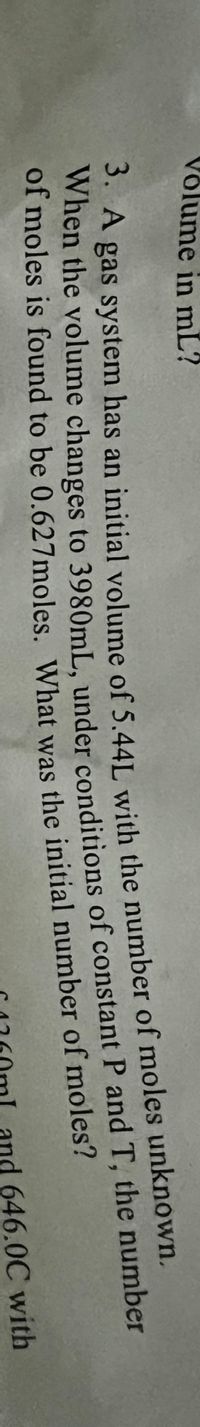
Transcribed Image Text:Võlume in mL?
WA gas system has an initial volume of 5.441 with the number of moles unknown.
when the volume changes to 3980mL, under conditions of constant P and T, the number
of moles is found to be 0.627moles. What was the initial number of moles?
3. А
When the to of P and T, the number
and 646.0C with
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The most common technique used in laboratory to determine the molar mass of a volatile liquid is.? A. Gravimetry B. Mass spectrometry C. None of the above D. Calorimetery E. Analytical Balancearrow_forwardWhat is the molarity of a solution that is composed of 2.5 moles of NaOH dissolved in enough water to make 1,255 of solution? (Report your answer rounded to two decimal places ) Show all workarrow_forwardPlease answer the three parts but have the answers match the parts not all in one answerarrow_forward
- Stoichiometry WS #1 (DA Review) 1. How many seconds are there in 2.5 years? 2. An average human heart beats 60. times per minute. If you live to be 82 years old, how many times will your heart beat?arrow_forwardNeed help with question 1arrow_forwardCreate a scenario would show the smallest deviation from an ideal gas (i.e., would show the most ideal behavior). Molar mass: ["", ""] Pressure: ["", ""] Temperature: ["", ""]arrow_forward
- Which of the following can be used to find moles in a stoichiometry calculation? а. The molarity of a solution and it's volume. b. The mass of a solid and it's molar mass. С. The pressure, volume, and temperature of an ideal gas. d. All of the abovearrow_forwardWhat is the mole-to-mole ratio between the two reactants? Select one: a. 2 mole CaCO3 to 3 mole NaCl b. 1 mole CaCO3 to 1 mole NaCl c. 1 mole Na2CO3 to 1 mole CaCl2 d. 1 mole CaCl2 to 1 mole Na2CO3 e. 2 mole Na2CO3 to 1 mole CaCO3arrow_forwardA 21 L reaction vessel contains only containts He gas. The system is kept at 45 ∘C and the pressure inside the vessel is found to be 0.435 atm. a) Find the number of moles of He in the reaction vessel. b find the number of moleculesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY