Question
Solve without using chatgpt and show work!!
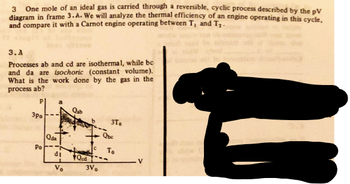
Transcribed Image Text:3
One mole of an ideal gas is carried through a reversible, cyclic process described by the pV
diagram in frame 3.A. We will analyze the thermal efficiency of an engine operating in this cycle,
and compare it with a Carnot engine operating between T, and T₂-
3.A
Processes ab and cd are isothermal, while be
and da are isochoric (constant volume).
What is the work done by the gas in the
process ab?
Qab
3po
3T
Qbc
Po
Qda
C
To
P
di
Qod
V
Vo
3V0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images
