
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
2s+3o2→2so3 if 13.1 grams of SO3 can be created and 5.8 grams were actually created, what is the percent yield
Transcribed Image Text:B Quizzes - 2021S2 Kennedy.T CHE X
b Search results for '25+302-2so3 x
G 25+302-2so3 if 13.1 grams of SC x
G how to screenshot on hp - Goog x
+
A instruction.gwinnett.k12.ga.us/d2l/Ims/quizzing/user/attempt/quiz_start_frame_auto.d21?ou=2594122&isprv=&drc=1&qi=2439923&cfql=0&dnb=0&fromQB=0
Spring 2021 Performance Final
Time Limit: 24:00:00
Time Left:16:26:15
Jesus Ortiz-Pecina: Attempt 1
Question 3 (3 points)
Page 2:
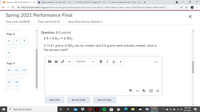
2 S + 3 02 --> 2 SO3
7
8
If 13.61 grams of SO3 can be created and 5.8 grams were actually created, what is
the percent yield?
--
--
9
--
Format
B I U
Page 3:
10
11
12
13
14
</>
Add a File
Record Audio
Record Video
8:03 PM
P Type here to search
O O G 4)
4/27/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If 0.460 mol of liquid CS2 and 0.500 mol of gaseous O2 are reacted stoichiometrically according to the balanced equation, how many moles of gaseous CO2are produced? CS2(l) + 3O2(g) → CO2(g) + 2SO2(g) Molar Mass (g/mol) CS2 76.143 O2 31.998 CO2 44.009 Density (g/mL) CS2 1.26 Molar Volume (L) 22.4 at STP Gas Constant(L.atm.mol-1.K-1) 0.0821arrow_forwardWhen 11.0 mol of NO reacts, the actual yield of N2 is 8.63 mol. What is the percent yield? 4NH3(g) + 6NO(g) --> 5N2(g) + 6H2O(l)arrow_forwardGiven the following balanced equation: N2+3H2 = 2NH3 1. If the actual yield of NH3 is 14g, what is the percent yield?arrow_forward
- Elemental silicon can be produced by the reduction of silicon dioxide, or sand, with carbon: SiO2 + 2 C → Si + 2 CO When 35.0 kg of SiO2 react with 25.3 kg of C, 14.4 kg of Si are recovered. What is the percent yield for the reaction?arrow_forwardNitric Oxide can be made from the oxidation of ammonia as shown below. A reaction between 8.5 g of NH3 and 25.0 g of O₂ produces 12.0 g of NO. What is the percent yield for this reaction? 4 NH3(g) + 5O₂(g) → 4 NO(g) + 6 H₂O(g) Molar masses (in g mol-¹): NH3 17.034, 0₂ 32.00, NO 30.01, H₂O 18.016arrow_forwardWhen 24.5 g of NH3 reacts, the actual yield of H20 is 26.5g. What is the percent yield? 4NH3(g) + 6NO (g) -> 5N2(g) + 6H20(l)arrow_forward
- If 18.2 kg NH3 is produced by a reaction mixture that initially contains 6.00 kg H2 and an excess of N2, what is the percent yield of the reaction? N2(g) + 3H2(g) → 2NH3(g)arrow_forwardIn the reaction 2H2 + O2 →2H2O 2H2 + O2 →2H2O If 2 grams of hydrogen is reacted with excess oxygen, and it produces 17.5 grams of water, the percent yield is 48.6 % True or False?arrow_forwardThe products of complete combustion of acetaldehyde CH 3 CHO are shown in the following unbalanced equation CH 3 CHO(l)+O 2 (g) CO 2 (g)+H 2 O(l) . This reaction has a CO 2 percent yield of 64.1% will be the mass of CO 2 experimentally obtained wien 49.1 O 2 and reacted? Molar masses: CH 3 CHO=4.052 g mol mol: H 2 O=18.016 approx molarrow_forward
- Dichlorine monoxide, Cl,0, is sometimes used as a powerful chlorinating agent in research. It can be produced by passing chlorine gas over heated mercury(I) oxide according to this equation: HgO+Cl2 → HgCl, + Cl20 What is the percent yield, if the quantity of reactants is sufficient to produce 8.90 g of Cl,0 but only 2.10 g is obtained? Answer in standard notation to the nearest tenth of a percentarrow_forwardFor the reaction SO3 + H2O→ H2SO4, calculate the % yield, if 343 grams of SO3 react with excess water to produce 104g H2SO4arrow_forwardAccording to the balanced reaction below, calculate the quantity of moles of NH3 gas that form when 4.20 mol of N2H. liquid completely reacts: 3 N2H4(1) → 4 NH3(g) + N2(g) X STARTING AMOUNTarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY