2H4O) is produced by catalytic oxidation of E (C2H4) but competes with an undesired combustion reaction: 2C2H4+02-2C2H4O C2H4+302-2CO2 + 2H₂O The reactor effluent is sent to a separation process that produces two streams: 1) the unreacted E and O and 2) the EO, water, and carbon dioxide products. The E and O are recycled and mixed with a fresh feed of E and O to form the reactor feed. Assume the reactor feed (not the recycle stream or fresh feed) contains 3 moles E per mole O and assume a basis of 100 mol/'s for the reactor feed. If the single-pass conversion of ethylene is 21.4% and 92.0% of the E that reacts forms EO. Determine the required flow of ethylene in the fresh feed (ne1). n1 DE=NE1 no=no1 100 n=75 no=25 n4 DEFNE4 no no4 Reactor n2 nene2 no=no2 nEO NEO2 Separator n3 DE=0 no=0 NEO-NEO3
2H4O) is produced by catalytic oxidation of E (C2H4) but competes with an undesired combustion reaction: 2C2H4+02-2C2H4O C2H4+302-2CO2 + 2H₂O The reactor effluent is sent to a separation process that produces two streams: 1) the unreacted E and O and 2) the EO, water, and carbon dioxide products. The E and O are recycled and mixed with a fresh feed of E and O to form the reactor feed. Assume the reactor feed (not the recycle stream or fresh feed) contains 3 moles E per mole O and assume a basis of 100 mol/'s for the reactor feed. If the single-pass conversion of ethylene is 21.4% and 92.0% of the E that reacts forms EO. Determine the required flow of ethylene in the fresh feed (ne1). n1 DE=NE1 no=no1 100 n=75 no=25 n4 DEFNE4 no no4 Reactor n2 nene2 no=no2 nEO NEO2 Separator n3 DE=0 no=0 NEO-NEO3
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
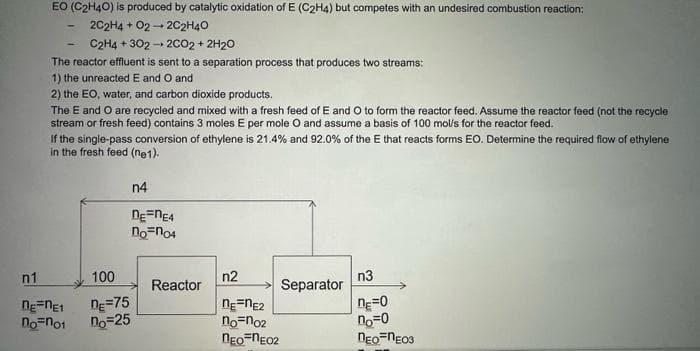
Transcribed Image Text:EO (C2H40) is produced by catalytic oxidation of E (C₂H4) but competes with an undesired combustion reaction:
2C2H4 + 02- + 2C2H4O
C2H4 +302 → 2CO2 + 2H20
The reactor effluent is sent to a separation process that produces two streams:
1) the unreacted E and O and
2) the EO, water, and carbon dioxide products.
The E and O are recycled and mixed with a fresh feed of E and O to form the reactor feed. Assume the reactor feed (not the recycle
stream or fresh feed) contains 3 moles E per mole O and assume a basis of 100 mol/s for the reactor feed.
If the single-pass conversion of ethylene is 21.4% and 92.0% of the E that reacts forms EO. Determine the required flow of ethylene
in the fresh feed (ne1).
n1
nε-ne1
no no1
100
De=75
no=25
n4
DE=NE4
non04
Reactor
n2
nε=nE2
no-no2
nEO-NEO2
Separator
n3
n=0
no=0
nEO-NEO3
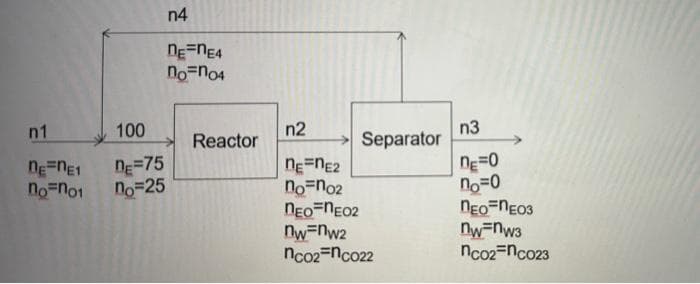
Transcribed Image Text:n1
100
nE=NE1
n=75
no no1 no 25
n4
nE ne4
no no4
Reactor
n2
Separator
ne=nE2
no no₂
NEO-NEO2
nw=nw2
nco2=nco22
n3
nE=0
no=0
NEO-NEO3
nw=nw3
nco2nc023
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 18 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The