
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
For the following reactions, determine the requested quantities based on the starting amounts of the given reactants. (Answers I have put on there so far are guesses.)
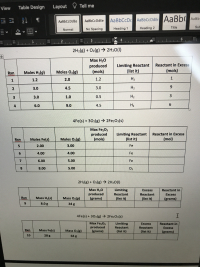
Transcribed Image Text:2H2(g) + O2(g) → 2H20(I)
Max H20
Limiting Reactant
(list it)
Reactant in Excess
produced
(mols)
Rxn
Moles H2(g)
Moles 02(g)
(mols)
1
1.2
2.0
1.2
H2
1
2.
3.0
4.5
3.0
H2
9.
3.0
1.0
0.5
H2
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemicals won’t react unless you have added the right ratios. If you are trying to create a product, you must add the correct amounts of reactants; otherwise it won’t form. When you balance an equation, it will inform you about how much of the reactant you need, which will enable you to guess how much product you will make. You must establish a ratio for the reaction to occur. In your opinion, which one of these reasons is the best? Justify your response. In your opinion, which of these reasons is the worst? Justify your response. Develop a different plausible explanation and share.arrow_forwardBalance the following chemical reaction. Enter the sum of the balanced coefficients as your answer. Assign "blank" coefficients a value of 1. ammonia + oxygen gas -→ nitrogen monoride + waterarrow_forward< For the following reaction, 62.5 grams of silver nitrate are allowed to react with 27.9 grams of copper(II) chloride. silver nitrate (aq) + copper(II) chloride (s) → silver chloride (s) + copper(II) nitrate (aq) What is the maximum amount of silver chloride that can be formed? [Review Topics] [References] Use the References to access important values if needed for this question. What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? Submit Answer Retry Entire Group Show Hint 9 more group attempts remaining grams grams Previous Next Sve and Exit Save and Exitarrow_forward
- The starting materials in a chemical reaction are called your "initial products". True False If the number of each type of atom on one side of the arrow differs from the number on the other side, the equation is still considered balance. True False Stoichiometry is the qualitative study of reactants and products in a chemical reaction. True Falsearrow_forwardBalance the following chemical equation by placing a coefficient (including 1) in each answer box that is in front of each reactant and each product. While coefficients that are equal to 1 are not placed in front of either a reactant or product, you MUST include any coefficient that is equal to 1 in this problem. type your answer... Re3P7- type your answer... Re + type your answer... P4arrow_forwardBalance the following chemical equation by placing a coefficient (including 1) in each answer box that is in front of each reactant and each product. While coefficients that are equal to 1 are not placed in front of either a reactant or product, you MUST include any coefficient that is equal to 1 in this problem. type your answer... C6H8+ type your answer... 0₂ type your answer... CO₂ + type your answer... H₂Oarrow_forward
- 3. Complete the following neutralization reactions. Word equations should be completed as word equations, and chemical equations should be completed as balanced chemical reactions. a. sulphuric acid + lithium hydroxide → b. KOH + H₂SO4 12 4. For one of the fuels in gasoline, pentane (CH2), complete the following combustion reactions. Make certain your equations are balanced. a. complete combustion C+0₁ H₂→ 5 12 2 b. incomplete combustion C+012 + H₂arrow_forwardBalance the following equations using lowest whole number coefficients. A number 1 must be placed where applicable.Example:H2O(l) → H2(g) + O2(g)is correctly balanced as2 H2O(l) → 2 H2(g) + 1 O2(g)You will record only the coefficients as your answer.Your answer should be221arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY