
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Please note all assumptions and when appendix values are used.
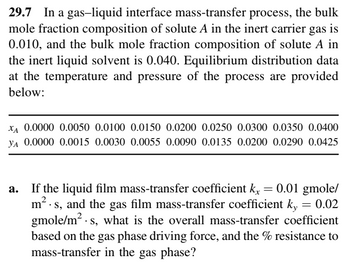
Transcribed Image Text:29.7 In a gas-liquid interface mass-transfer process, the bulk
mole fraction composition of solute A in the inert carrier gas is
0.010, and the bulk mole fraction composition of solute A in
the inert liquid solvent is 0.040. Equilibrium distribution data
at the temperature and pressure of the process are provided
below:
XA 0.0000 0.0050 0.0100 0.0150 0.0200 0.0250 0.0300 0.0350 0.0400
YA 0.0000 0.0015 0.0030 0.0055 0.0090 0.0135 0.0200 0.0290 0.0425
a. If the liquid film mass-transfer coefficient k = 0.01 gmole/
m². s, and the gas film mass-transfer coefficient ky = 0.02
gmole/m². s, what is the overall mass-transfer coefficient
based on the gas phase driving force, and the % resistance to
mass-transfer in the gas phase?

Transcribed Image Text:b. If the liquid film mass-transfer coefficient is still kx = 0.01
gmole/m². s, what is the new value of ky required to make
the process 10% gas phase mass-transfer controlling?
C.
Plot (XA, YA,i) on the equilibrium line in yA - XA coordinates
for parts (a) and (b), and compare results.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Similar questions
- PLEASE ANSWER QUESTION WITH RIGHT ANSWERS, ANSWERS ATTACHED FEEDBACK ATTACHED ALSO DONT COPY AND PASTE FROM OTHER ANSWERED QUESTIONS AS THESE ARE WRONGarrow_forwardWhy is a linear calibration curve important? Couldn't interpolation be used to predict values within the calibration range?arrow_forwardDiscuss any one the Pharmaceutical product with the following titles. Raw materials Flow diagram Reactions Process description Process conditions Usesarrow_forward
- Write out the steps (including equations) for BUBL P, DEW P, BUBL T, and DEW T calculations using the modified Raoult’s law.arrow_forwardUsing the data below, construct a calibration curve. Include the error bars and the equation of the line and the linearity (R2) of the curve. Calcium concentration (ppm) (X-data) Absorbance (Y-data) Run 1 Absorbance (Y-data) Run 2 Absorbance (Y-data) Run 3 0.3 1109 1069 1155 1.2 1225 1168 1233 2.1 1472 1319 1389 4.2 1497 1523 1472 8.0 1833 1898 1849 16 2066 2012 2051arrow_forwardDescribe k-ε model with its advantages and disadvantages.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The