
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
Convert
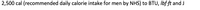
Transcribed Image Text:2,500 cal (recommended daily calorie intake for men by NHS) to BTU, Ibf ft and J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Three types of fluids stack in an open tank as shown in the plot. Select the expression of the gage pressure at location 1, P1(gage)=______ A. Patm B. 0 C. 101.325 kPa D. It can not be determined.arrow_forwardA gas has a density of 0.094 lb/ft3 at 100 F and 2atm. What pressure is needed to change the density to 0.270 lb/ft3 at 250 F A. 7.28 atm B. 6.32 atm C. 3.45 atm D. 5.25 atmarrow_forward1. If 1 gallon of oil = 1.4 therm, how many gallons have been wasted by heat loss in Feb? (1 therm = 100,000 BTU)_____________________________________________________________________________________2. If $1.30 per therm, how much did you waste from house heat loss in Feb?arrow_forward
- 7. For ethanol, a handbook gives: sp. gr. 60°F = 0.79389. What is the density of ethanol at 60°F? 8. The specific gravity of steel is 7.9. What is the volume in cubic feet of a steel ingot weighing 4000 lb? 9. The specific gravity of a solution is 0.80 at 70°F. How many cubic feet will be occupied by 100 lb of the solution at 70°F?arrow_forward1. One pound of an ideal gas undergoes an isentropic process from 95.3 psig and a volume of 0.6 ft to a final volume of 3.6 ft. If Cp=0.124 and Cy=0.093 BTU/Ik R. What are a. T2 b. P2 c. AH d. Warrow_forwardHand by written ans.Calculate the amount of dissolved oxygen in mg/L in river water at 30°C under saturated conditions at an atmospheric pressure of 100 kPa (Assume density of water = 1000 kg/m3 ) Select one: a. 5.55mg/L b. 7.77mg/L c. 6.66mg/L d. 8.88mg/Larrow_forward
- Problem: Determine the pressure in kPa at the following points: a) Point 1 b) Point 2 c) Point A Fluid from Point 1 to Point 2 is mercury (Hg) with Specific Gravity of 13.6. Fluid from Point 2 to Point A is water (H2O). Point 2-A (h=12 cm) open to atm -H20 48 cm - 4. Hg (S.G.= 18.6)arrow_forwardThermodynamics problem. While at sea level the atmospheric pressure is 100 kPa, at the summit of Mount Everest, the pressure is reduced to 35 kPa. a) What is the saturation temperature of water? What is the specific volume of water in liquid and gaseous state?arrow_forwardA2: FLUID FLOW, FLOW RATE & PRESSURE DROPProblem:1. Determine the specific gravity of the liquid with a mass of 1,200 kg/m3.arrow_forward
- 7. If the only change was to remove the air pressure, what will happen to the pressure? Pressure Pressure 121.0 kPa A. increase by 101.3 kPa B. decrease by 101.3 kPa C. stay the same D. Something else 7. Prediction and explanation with supportarrow_forward1. A driver fills an 18.9L steel gasoline can with gasoline at 15 deg C right up to the top. He forgets to replace the cap and leaves the can in the back of his truck. The temperature climbs to 42 deg C by 1P.M. how much gasoline spills out of the can?arrow_forwardB. How deep can a diver descend in ocean water without damaging his watch, which will withstand an absolute pressure of 5.5 bar? Pwater 1025 kg/marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY