
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Determine the number of protons, neutrons, and electrons in the following: (Also attached in the photo)
25
x
12
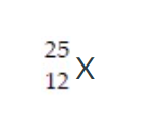
Transcribed Image Text:The image features a simple mathematical expression involving multiplication. The expression is written with one number above the other, separated by a multiplication sign "X". Specifically, the numbers and symbol appear as follows:
25
X
12
This suggests that 25 is being multiplied by 12.
Understanding this format is essential for basic arithmetic operations, particularly multiplication. This setup is traditionally used to perform long multiplication manually. Here's a step-by-step guide to multiplying these two numbers using long multiplication:
1. Write the numbers in a vertical format with the larger number on top:
```
25
x 12
```
2. Multiply the digit in the ones place of the bottom number (2) by each digit of the top number, starting from the right:
```
25
x 12
-----
50 ← (2 * 25)
```
3. Place a zero below the first line since we're moving to the tens place (1 in 12 is actually 10):
```
25
x 12
-----
50
250 ← (1 * 25 moved one place to the left)
```
4. Add the results of the two multiplications:
```
25
x 12
-----
50
250
-----
300
```
So, \(25 \times 12 = 300\).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following has the greatest number of neutrons? O antimony-123 O tin-121 55 Fe 26 O the Period 5 halogen with a mass number of 123arrow_forwardFill in the information missing from this table. nuclide protons neutrons Z A 12 C ☐ ☐ 13 ☐ 27 ☐ ☐ 30 38 5arrow_forwardThe total mass of 12 protons, 27 neutrons, and 24 electronsarrow_forward
- An atom has a diameter of 3.00 Å and the nucleus of that atom has a diameter of 7.50 × 10-5 Å. Determine the fraction of the volume of the atom that is taken up by the nucleus. Assume the atom and the nucleus are a sphere. fraction of atomic volume: Calculate the density of a proton, given that the mass of a proton is 1.0073 amu and the diameter of a proton is 1.69 × 10-¹5 m. density: g/cm³arrow_forwardFill in the information missing from this table. nuclide protons neutrons 15 16 66 158 75 ninarrow_forwardHow many protons, neutrons, and electrons, respectively, are in 57Co2+?arrow_forward
- Find the number of electron and neutrons in the following: 52Cr+2 Electrons Neutronsarrow_forwardHow many protons, neutrons and electrons are there in a neutral atom of the isotope with the nuclear symbol: 90 Y 39 protons: neutrons: electrons:arrow_forwardFind the number of electron and neutrons in the following: 195Pt+2 Electrons Neutronsarrow_forward
- The mass of a single uranium atom is 4.70×10-22 grams. How many uranium atoms would there be in 196 milligrams of uranium?arrow_forwardUranium-238 undergoes radioactive decay until a stable isotope is reached. One of the steps is shown below. The chief would like to know what new elements are being produced. Determine the atomic mass, atomic number, and symbol of the new element based on the equation below. 4 227 Ac → ? + ½ ½ a >? 2 89 Atomic Mass Atomic Number Symbol Karrow_forwardAn aluminum atom has an average diameter of about 3.0 * 10- 8 cm. The nucleus has a diameter of about 2.0 * 10- 13 cm. Calculate the ratio of the atom’s diameter to its nucleus.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY