
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
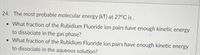
Transcribed Image Text:24. The most probable molecular energy (kT) at 27°C is.
• What fraction of the Rubidium Fluoride ion pairs have enough kinetic energy
to dissociate in the gas phase?
• What fraction of the Rubidium Fluoride ion pairs have enough kinetic energy
to dissociate in the aqueous solution?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The water gas shift reaction is represented by the equation and enthalpy change shown below: co + H20 = CO2 + H2 AH'R = -41 kJ mol1 For this question answer the following questions: i. What does the phrase 'equilibrium limited' mean? ii. What impact would increasing temperature have on the position of this equilibrium? i. How would adsorbing Co, on a solid adsorbent affect this equilibrium? Write your answer as i= ii = iii =arrow_forwardA reaction chamber is sealed, but connected to heating elements to change the temperature of the system. What kind of system is this? arrow_forward1. When solid magnesium phosphate is in equilibrium with its ions, the ratio of magnesium ions to phosphate ions is:arrow_forward
- The equilibrium constant changes when... a a catalyst is added. b the temperature changes. c the surface area changes. d the concentration of reactants changes e more than one above is correctarrow_forwardIf the equilibrium constant for a reaction is very large, what is the reactionarrow_forwardWhen solid lead(II) phosphate is in equilibrium with its ions, the ratio of lead(II) ions to phosphate ions is which of the following? 2:1 3:2 1:1 2:3 1:2arrow_forward
- Use Le Châtelier’s Principle to explain the effect each of the following changes will have upon the system—will the equilibrium shift toward the product or reactant side? Why? N2(g) + 3 H2(g) ↔ 2 NH3(g) + heat a. If more hydrogen is added to the system the equilibrium will shift to the..... (pick one and explain)i. Right ii. Left iii. Remains unchanged b. If ammonia is removed from the system the equilibrium will shift to the..... (pick one and explain)i. Right ii. Left iii. Remains unchanged c. If nitrogen is removed from the system the equilibrium will shift to the..... (pick one and explain)arrow_forward4. Which of the following statements is false? A. Increasing the volume of the container will couse the reaction to shift to the left. B. Decreasing the volume of the container will cause the partial pressure of N2 to increase. C. Adding an inert gas such as Neon will couse the pressure in the container to increase. D. Decreasing the partial pressure of NO will cause the partial pressure of N2 to increase. E. The addition of a catalyst will speed up the reaction but will have no effect on the position of equilibrium.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY