
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Base peak structure for the compond below
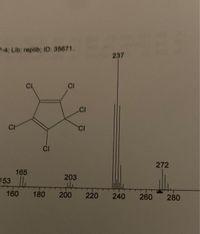
Transcribed Image Text:4 Lib: replib; ID: 35671.
237
CI
CI
.CI
Ch
CI
CI
272
165
203
153
160
180
200
220
240
260
280
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- exe/lo_u-lgNsikr7j8P3jH-IQs_dp5pR4ENzvdYC-70kXyMz36BqJhw3sVPj_jpaFLxvGArYxlbmcyqa71YYPJBG6RjdYAdGPjGhFLILID-HEX1YcqAB?1oBw7QYjlbavbSPXtx-YCjsh_7mMmrq#item Course Home Login | Student Veri... Bb Logout MyrogrammingLab Imported From IE ITEC2110:Summer2.. TunesToTube - Upl.. Web Development. Publix App O KINETICS AND EQUILIBRIUM Using the Arrhenius equation to caiculate k at one temperature fr.. Ci The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E = 54.0 kJ/mol. If the rate constant of this 1 -1 at 11.0 °C, what will the rate constant be at -16.0 °C? reaction is 0.012 M Round your answer to 2 significant digits. -1 -1 x10arrow_forwardAICI3 సిం ఇయి. a)arrow_forwardo/index.html?deploymentld%3D55750828934189288909969212&elSBN=9781305657571&snapshotld%3D2199... Tp * NDTAP Q Search this co Use the References to access important values if needed for this question. Cyclopentasilane (SigH10) is a liquid with a density of 0.963 g cm3. It reacts with oxygen to give silicon dioxide (SiO2) and water. Calculate the mass of water that would form if 29.4 cm³ of cyclopentasilane reacted completely with excess oxygen. g water Submit Answer 4 question attempts remainingarrow_forward
- - Jasmine Bassler islexe/1o_u-IgNslkr7j8P3jH-lvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym9hTWHc1BKL64lfbHd6ekurSoiptYikmiZnHbvsb80JQdwhEkLV6006Tp3Ecq?1oBw7QYJibavbSPXtx-YCjsh_7mMmrq#iter O CHEMICAL REACTIONS 三I Dilution Jasm A chemist mnakes 380. mL of calcium sulfate (CASO, working solution by adding distilled water to 240. mL of a 5.39 mM stock solution of calcium sulfate in water. Calculate the concentration of the chemist's working solution. Be sure your answer has the correct number of significant digits. | mM 1IIarrow_forward2+ d. 4H3O+ (aq) + 2Cl(aq) + MnO₂ (s) ⇒ Mn²+ (aq) + 6H₂O(1) + Cl₂ (9) Oke O Ke = = O Ke = 2+ [Mn²+ ][C1₂] [H3O+] * [CI-1² [Mn²+][H₂O][C1₂] 2+ [H³O+][CI¯]²[MnO₂2] [Mn²+ ] [H₂O1] [C1₂] 2+ [H3O+] * [CI-1²[MnO₂]arrow_forwardلا © Macmillan Learning H H-C-OH H₂/Pt HO-C-H $ % do LO 5 H₂C-OH OCT 11 <6 Y MacBook Pro Resources Q2Q Erase Select Draw Templates More /// C 0 H G c 2Q & * ( 7 8 9 0 ) U 0 P SUDarrow_forward
- Blackboard | Miami D x M CHM 1046L_Week 9 x S HOL - Instance A ALEKS - Daniela GALL x Blackboard| A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslikr7j8P3jH-liGmxpvVN4ENzvdYC-70kXyMz36BqJhw3sVP20GT MEASUREMENT AND MATTER Simplifying unit expressions Rewrite this measurement with a simpler unit, if possible. kg-m 4.7 2 m m Note: If you can simplify the unit at all, it may be possible to make more than one simplification. Be sure ロ.口 olaarrow_forward30 70- 60- 50- 40- 30 20 10 0₁ 4000 Ророжа Structue nd, C₂H₂1N 3500 иде Sp 3000 2500 18 +2-20 го- 2000 1800 hu 1600 Ф тро 1400 1200 1000 800 6 Н 6 Н 9 Н 600 Уarrow_forwardAssessment-Chem 18-Gen (X 80 $ 54 R UCI General Chemistry Peer Tu X 65.2% Use the molar bond enthalpy data in the table to estimate the value of AHin for the equation CCI, (g) + 2 F₂ (g) →→→ CF₂(g) + 2Cl₂(g) The bonding in the molecules is shown. ΔΗ; = 888 F4 ++++ % 5 FS F-F A 6 New Tab MacBook Air ܀ T Y F6 & 7 CICI CI-CI A4 F7 * 8 ∞ Resources Average molar bond enthalpics. (Hond) kJ. mol Bond 464 C=N 142 N-H 351 N-N 502 N=N 730 N=N 347 F-F 615 811 414 439 331 276 293 615 Bond O-H 0-0 C-0 0=0 C=0 C-C C=C C=C C-H C-F C-CI C-Br C-N C=N DII FB 69 DD F9 kJ Question Source: McQuarrie, Rock, And Gallogly 4e-General Chemistry Publisher: University Science Books 1 0 ) CI-CI Br-Br H-H H-F H-CI H-Br H-S S-S 0 A F10 I' Check Answer P kJ. mol-1 890 390 159 418 945 155 243 192 435 565 431 368 364 225 3 F11 0 + Show All 411) F12 X }arrow_forward
- d ← Chrome esc e 24% (92) ALE X Mathwa X G 102 cels X ☆ www-awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNsikr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHinMCnqLGstAPM iZtogOAPGJIWPnLIY... 17.4 Solubility and... 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... Y SOLUTION: The le... Math 115 W-S Fall.... File email d x Edit View History Bookmarks Profiles View 7. Q A Nancy X Z Explanation O KINETICS AND EQUILIBRIUM Using the Arrhenius equation to calculate k at one temperature... 2 W S The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E-29.0 kJ/mol. If the rate constant of this reaction is 2.8 x 10³ Ms at 320.0 °C, what will the rate constant be at 222.0 °C? -1 Round your answer to 2 significant digits. Check X A ALEKS X CIChego X H command # 3 E Tab Window Help D $ 4 C X R > F 5 (92) ALE X % 5 V I T G #tv Welcom X A ALEKS ^ 6 MacBook Pro B Y We 9 "/ & 7 H X U N You 8 J 0000015 1…arrow_forwardCou X A Ch 6 x C Sear X * Sear X Sear x b > Hov x2 Con x Con X b Sear X y! cher X M Inbc X M Inbc X + Vent X 8 https://www.saplinglearning.com/ibiscms/mod/flcn/view.php?id3D15033867 + Sapling Learning macmillan learning Ch 6 Homework Jason Bauer , Sapling Learning > Ventura College - Chem V30 (31510) - Spring21 - ALAWDI > Activities and Due Dates > Ch 6 Homework E 20 of 24 Questions O Assignment Score: O Resources O Hint 68.3% Check Answer O 16 Question 100% x2 Question 20 of 24 > 1 of 5 Attempts Correct How many moles of CaCl2 are in 7.76 x 1024 formula units? O 17 Question 100% x2 1 of 5 Attempts Correct 7.76 x 1024 formula units = mol O 18 Question 100% x2 2 of 5 Attempts Correct O 19 Question 1 of 5 Attempts 100% x2 Correct 20 Question 0% O of 5 Attempts 21 Question 0% x2 O of 5 Attempts © 2011-2021 Sapling Learning, Inc. about us careers privacy policy terms of use contact us help 1:42 PM P Type here to search 99+ 2/18/2021 19arrow_forwardᴡʀɪᴛᴇ ᴛʜᴇ ᴄᴏᴍᴘʟᴇᴛᴇ ʙᴀʟᴀɴᴄᴇᴅ ᴇQᴜᴀᴛɪᴏɴꜱ ᴏꜰ ᴛʜᴇ ꜰᴏʟʟᴏᴡɪɴɢ ᴄʜᴇᴍɪᴄᴀʟ ʀᴇᴀᴄᴛɪᴏɴꜱ. ꜱʜᴏᴡ ʏᴏᴜʀ ꜱᴏʟᴜᴛɪᴏɴarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
