
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Can someone clearly explain the answer to the attached?
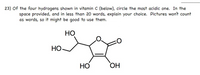
Transcribed Image Text:**Problem 23: Identifying the Most Acidic Hydrogen in Vitamin C**
**Question:**
Of the four hydrogens shown in vitamin C (below), circle the most acidic one. In the space provided, and in less than 20 words, explain your choice. Pictures won’t count as words, so it might be good to use them.
**Diagram Description:**
The diagram depicts the molecular structure of vitamin C. It is a cyclic compound with four hydroxyl (OH) groups attached to the ring structure. Each OH group has one hydrogen atom.
**Explanation Tip:**
Consider which hydrogen is bonded to the most electronegative atom and is stabilized by resonance or any other effect that makes it more acidic.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- CHE 103 Recitation Problems x A Review for Quiz IV.docx: CHE X A Review for Exam IlI.docx: CHE X b Draw the structural formula O chem prob 15.48.pdf G how to screenshot on LG gra x + O File | C:/Users/briannawend/Downloads/chem%20prob%2015.48.pdf E Apps G Google YouTube Netflix |h Hulu Home | My CedarCr.. ii Handshake b My Questions | bart. 2 13 River Rd, Oak Ri.. p. Classic Szarlotka: P.. st Pirates Perch - 6 BR.. Bookmarks Other bookmarks *hemic a cCTAT 15.48 1denthify funçional groups HO CH2OH C=0 HgC chem prob 15.48.pdf Show all PDFarrow_forwardHelp, what is the name molecule?arrow_forwardwhat is the molecular fomula for this pleasearrow_forward
- can you answer this again in a more understanding and easy to interpret way. Like an answer key.arrow_forward:&:&:&&;&:&/&arrow_forwardWithout doing any calculations, predict the sign of ΔH foreach of the following reactions:(a) 2 NO2(g)--->N2O4(g)(b) 2 F(g)------>F2(g)(c) Mg2+(g) + 2 Cl-(g)---->MgCl2(s)(d) HBr(g)----->H(g) + Br(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY