
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Pls help ASAP
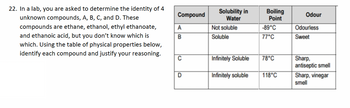
Transcribed Image Text:22. In a lab, you are asked to determine the identity of 4
unknown compounds, A, B, C, and D. These
compounds are ethane, ethanol, ethyl ethanoate,
and ethanoic acid, but you don't know which is
which. Using the table of physical properties below,
identify each compound and justify your reasoning.
Compound
A
B
C
D
Solubility in
Water
Not soluble
Soluble
Infinitely Soluble
Infinitely soluble
Boiling
Point
-89°C
77°C
78°C
118°C
Odour
Odourless
Sweet
Sharp,
antiseptic smell
Sharp, vinegar
smell
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 19 Draw a diastereomer for the following monosaccharide. Upload your answer in the last question. сно H- HO- H- HO- ČH2OH Edit View Insert Format Tools Table 12pt v Paragraph v I U Av D. MacBook Pro Search or type URL li #3 & 3 4 7 E Y U * 00 < CO O LOarrow_forwardO words Question 18 Draw the enantiomer for the following monosaccharide. Upload your answer in the last question. ÇH,OH H- -HO- H- -HO- ČH2OH Edit View Insert Format Tools Table 12pt v Paragraph v B IUA O words MacBook Proarrow_forwardPlease help me answer this table thank you.arrow_forward
- HNDglag ) tNaOHCag)Naloag Caq)+ HoO Darrow_forwardActivities acromolecules Fill in the table below to review macromolecules and their components (monomers). Macromolecule Examples Examples Macromolecules Components methionine, tyrosine, alanine. etc. (Polymer) Protein Nucleic Acid Carbohydrate 1 3 5 Components (Monomers) 2 Nucleotides 6 47 ofarrow_forwardproteins 1 The sequence of amino acids an example of a, protein structure. Select an answer and submit. For keyboard navigation, use the up/down arrow keysto select an answer. a. primary bi secondary tertiary Unansweredarrow_forward
- Br вгик - > Doxarrow_forwardQuestion 4 of 23 > Monosaccharides Disaccharides Polysaccharides Answer Bank CH,OH CH,OH HOCH2 OH CH2OH но HO, CH,OH CH,OH CH, CH,OH OH OH OH OH H он 0- он CH2OH О он CH2OH OH OH OH O. OH OHarrow_forwardBiological Macromolecules Identifying and drawing peptide bonds Draw the structure of threonylmethionine, a dipeptide made from threonine and methionine, as it would appear at physiological pH. $ 4 Explanation % 5 Click and drag to start drawing a structure. Check 6 MacBook Pro & 7 * 00 8 ( 9 X O 0:0 3 0 è D ▬▬ Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | O + 11 Ⓡ 2/5 =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY