
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
When a nucleus of emits an alpha particle, what are the atomic number and the
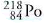
Transcribed Image Text:218
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A radioactive sample studied in a lab is found to have a half-life of 25 hours. If the initial sample contained 7.5x10¹⁰ radioactive nuclides estimate the number of radioactive nuclides present in the sample after 10 hours.arrow_forwardA radioactive sample has a half-life of 28 mins. If the initial amount of sample was 2 g, estimate how much will be left after 40 mins,arrow_forward- F0,0244t where An is the initial amount present and A is the amount present at time tin years Strontium-90 is a radioactive material that decays according to the function A(t)= Ag e Assume that a scientist has a sample of 500 grams of strontium-90. (a) What is the decay rate of strontium-90? (b) How much strontium-90 is left after 40 years? (c) When will only 300 grams of strontium-90 be left (d) What is the half-life of strontium-90? Question Viewer (a) The decay rate of strontium-90 is %. (Type an integer or a decimal. Include the negative sign for the decay rate.) (b) Approximately grams of strontium-90 is left after 40 years. (Do not round until the final answer. Then round to the nearest whole number as needed.) (c) Only 300 grams of strontium-90 will be left in about (Do not round until the final answer. Then round to the nearest tenth as needed.) years. (d) The half-life of strontium-90 is approximately years, (Do not round until the final answer. Then round to the nearest…arrow_forward
- A 60-kg person accidentally ingests a small source of alpha particles (RBE=15). The activity of the source is 0.04 Ci, the half-life of the source is 110 years, and each alpha particle emitted has an energy of 0.586 MeV. It takes 12hours for the alpha source to pass through the person’s digestive system and exit the body. How many alpha particles are absorbed by the person (assume that 100 percent of the alpha particles emitted by the source are absorbed by the person)? How much energy, in Joules, is deposited in the person by the source? What is the absorbed dose in rad? What is the absorbed dose in rem?arrow_forwardA banana contains the naturally occurring radioactive isotope potassium K-40 which undergoes Beta minus decay. Write the Beta decay formula to include the daughter isotope and the ejected particles.arrow_forwardGive good explanation Asap Thanksarrow_forward
- Q3: The table shows two isotopes of potassium and two isotopes of calcium. Type of emission Mass of neutral atom / u Number of electrons Number of protons Number of neutrons Total mass/u Mass defect/ u Binding energy / J Mass of the electron = 0.000549 u Mass of the proton = 1.007276 u Mass of the neutron = 1.008665 u TI ***** LEG 39K Stable 38.96371 SE nda 42K ß decay a) Use data about potassium-42 to explain the concept of mass defect. 3000 41.9624 19 19 23 42.34797 0.38557 5.76 x 10-11 De RAP 39 Ca ßt decay 38.9707 440 **** ****** D 42 Ca stable 41.95863arrow_forwardWhat isotope is produced when Np-239 emits a beta particle?arrow_forwardElements that appear in the same column of the periodic table often share similar chemical properties. In the case of the alkaline earth metals, this is troublesome since the body treats calcium (necessary for proper bone growth) and radium (a radioactive element) as chemically similar, storing both in bone marrow. The radium then bombards nearby bone cells with alpha particles, causing them to "crumble." Radium poisoning investigations often center on the identification of radium and its isotopes in bone samples using lonized isotope a mass spectrometer. Pictured is a schematic of a simplified mass spectrometer that shows the paths of calcium isotopes, barium (another alkaline earth metal) isotopes, and radium isotopes entering the chamber. The region shown is immersed in a constant magnetic field of 0.552 T pointing out of the plane of the schematic. Motion of the positively-charged isotopes toward the right was initiated by a potential A -AV- B difference of 3082 V on the two plates…arrow_forward
- If 88 (atomic number) 228 (atomic mass) ?? is produced, determine the parent isotope for each of the following types of decay and justify your answer. a) alpha decay b) beta negative decay c) beta positive decayarrow_forwardWhen a nucleus undergoes beta decay, _____. When a nucleus undergoes beta decay, _____. its atomic number increases by 1 it emits a proton it emits a high-energy photonarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON