
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
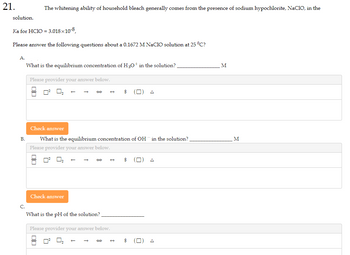
Transcribed Image Text:21.
solution.
Ka for HC1O = 3.018x10-8,
Please answer the following questions about a 0.1672 M NaClO solution at 25 °C?
A.
B.
C.
The whitening ability of household bleach generally comes from the presence of sodium hypochlorite, NaCIO, in the
What is the equilibrium concentration of H3O+ in the solution?
Please provider your answer below.
0²
Check answer
OC
→
→
Check answer
What is the equilibrium concentration of OH in the solution?
Please provider your answer below.
What is the pH of the solution?
Please provider your answer below.
$
$
M
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. A saturated solution of Mg(OH)2 is prepared having a large excess of Mg(OH)2. Sn(NO3)2 is added to the solution. Ksp = 2.0 ✕ 10-11 for Mg(OH)2 and Ksp = 5.3 ✕ 10-26 for Sn(OH)2. a) What [Sn2+] is required to start the precipitation of Sn(OH)2? b) What [Sn2+] is required so that the [Mg2+] in solution will be 0.12 M? 2. Solid NaI is added to a solution which is 0.025 M in TlNO3 and 0.015 M in AgNO3. Assume the volume remains constant. Ksp = 8.9 ✕ 10-8 for TlI and Ksp = 1.0 ✕ 10-16 for AgI. a) Which compound precipitates first TII or AgI? b) What is the concentration of the first ion precipitated when the second ion starts to precipitate? 3. What is the molar solubility of PbSO4 in 0.19 M MgSO4? Ksp = 1.0 ✕ 10-8 for PbSO4.arrow_forwardWhat hydroxide concentration is required to initiate precipitation of Al3+ from a 2.90x10-2 M solution of Al2(OH)3? The solubility-product constant for Al(OH)3 is 3x10-34. M lower the Al3+ concentration in the foregoing solution to 4.20x10-7 M? Marrow_forwardConsider a solution of calcium ion and oxalate ion ate equilibrium with solid calcium oxalate. Explain what would happen to the amount of solid if the following stress was added. Show all secondary reaction if happened. Ca2+(aq) + C2O42- (aq) CaC2O4(s) a. Adding OH- solution, Ca(OH)2(s) formed b. Adding H+ solution, H2C2O4(aq) formed.arrow_forward
- A solution contains 1.37x10-2 M zinc nitrate and 1.29x10-2 M barium acetate. Solid sodium carbonate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of carbonate ion when this precipitation first begins? [CO3²-] = Marrow_forwardExpress the equilibrium constant (K) for the chemical equations. (Note the physical states) A.) 2 KClO3 (s) ↔ 2 KCl (s) + 3 O2 (g) B.) HF (aq) + H2O (l) ↔ H3O+ (aq) + F- (aq) C.) NH3 (aq) + H2O (l) ↔ NH4+ (aq) + OH- (aq)arrow_forwardCS 10 nousT200 7. Tooth enamel (Ca5(PO4)3OH) is a slightly soluble compound that exists as the equilibrium shown below. If the Ksp of tooth enamel in water is 6.8 x 10-37 calculate the molar solubility of tooth enamel. a. b. 2+ 3- Cas(PO4)3OH (s) → 5Ca²+ (aq) + 3PO4 (aq) + OH- (aq) 2.7 x 105 M 5.84 x 10-8 M C. 8.2 x 10-¹9 M d. 6.4 x 109 M 2.00arrow_forward
- The following reaction represents which type of reaction? Na2SO4(aq) + BaCl2(aq)---> 2NaCl(aq) + BaSO4(s) a. Redox b. Titrationc. Neutralizationd. Displacement/Precipitation e. Dissolutionarrow_forward1. Calculate the [H+] and the pH of a buffer solution that is 0.10 M in CH3COOH (Ka = 1.8 x 10-5) and 0.20 M in CH3COONa 2. A solution of sodium hypochlorite has a pH of 5.20. Find the ratio [OCl-]/[HOCl] in this solution (Ka = 3.8 x 10-5) 3. What is the pH of a buffer made by mixing 1.00 L of 0.020 M benzoic acid, HC7H5O2, with 3.00 L of 0.060 M sodium benzoate, NaC7H5O2? The Ka for benzoic acid is 6.3 x 10-5arrow_forwardBUFFERS, HYDROLYSIS OF SALTS 1. A 0.0250 M Ca(X)2 has a pH of 8.049. Ca(X)2 is composed of a Ca2+ cation and an unknown anion X™, which undergoes hydrolysis. A. Write the balanced dissociation reaction of Ca(X)2 into its ions. Write your answer on your solution sheet B. Write the balanced hydrolysis reaction of the unknown anion, X. Write your answer on your solution sheet C. Calculate the Kh or Kp of X. D. Given the following Ką values of different weak acids, determine the identity of X. Weak acids Ka HIO 2.00 x 10-11 HCN 6.17 x 10-10 CH3COOH 1.80 x 10-5 HNO2 4.00 x 10-4arrow_forward
- Calculate the concentration (molarity) of Pb2+ ions in a solution made by adding 0.010 moles of Pb(NO3)2 to 1.0 L of a 2.0 M NaI solution. (Kf = 3.0 x 104 for PbI42−) - 4.9 x 10−2 -3.3 x 10−5 - 2.1 x 10−7 -1.7 x 10−7 -2.3 x 10−8arrow_forward5) Find the concentration of H30*(aq) in a 1.75 M solution of lactic acid, HC3H5O3, at 25°C. Ka= 1.38 x 10*. 6) Write the equilibrium expression for the ionization of HOI, and calculate the concentration of HOI(aq) in solution if [H3O*]=2.3 x 10° M and pKa = 10.7 at 25°C.arrow_forwardTitrating ascorbic acid with iodine is what type of titration? HO. HO. + 2 HI HO 3x-3x= h + HO HO OH Iodine Ascorbic Acid Dehydro-Ascorbic Acid O An acid-base neutralization titration, because iodine is a strong acid OA redox titration, because iodine acts as an oxidizing agent OA redox titration, because iodine acts as a reducing agent An acid-base neutralization titration, because ascorbic acid is a weak basearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY