
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
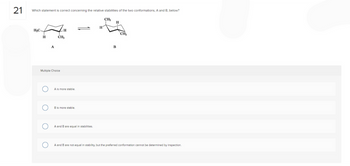
Transcribed Image Text:21
Which statement is correct concerning the relative stabilities of the two conformations, A and B, below?
H₂C
H
O
A
Multiple Choice
O
H
CH₂
A is more stable.
B is more stable.
A and B are equal in stabilities.
CH₂
H
B
CH₂
A and B are not equal in stability, but the preferred conformation cannot be determined by inspection.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank these conformations from least to most stable.arrow_forward40. Beginning with the Newman projection below as number 1, draw each of the six conformations for the molecule. Keep the back carbon static and rotate the front one clockwise. Number each drawing and rank them from the most stable to the least stable. Br H, Brarrow_forwardConsider 1,2-dimethylcyclohexane. a.Draw structures for the cis and trans isomers using a hexagon for the sixmembered ring. b. Draw the two possible chair conformations for the cis isomer. Which conformation, if either, is more stable? c. Draw the two possible chair conformations for the trans isomer. Which conformation, if either, is more stable? d.Which isomer, cis or trans, is more stable and why?arrow_forward
- Draw a perspective representation of the most stable conformation of 3-methylhexane. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars. 0 + 7 C Akri C H 12D EXP CONT Marvin JS by ChemAxon 1 H CI P UNOS Jarrow_forwardWhich of the shown dibromocyclohexanes are constitutional isomers? Br Br CI Br ČI Br ČI Br C 1 and 4 O1. 2 and 4 2. 2 and 3 O 3. 4. 1 and 3 1 and 2 5.arrow_forwardIdentify the correct chair conformations of the following compound and then indicate which one is more stable. OH OH OH HO 1 || 4 OI and II are correct chair structures and I is most stable OI and II are correct chair structures and II is most stable OI and III are correct chair structures and III is most stable O II and III are correct chair structures and II is most stable O II and III are correct chair structures and III is most stable |||arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY