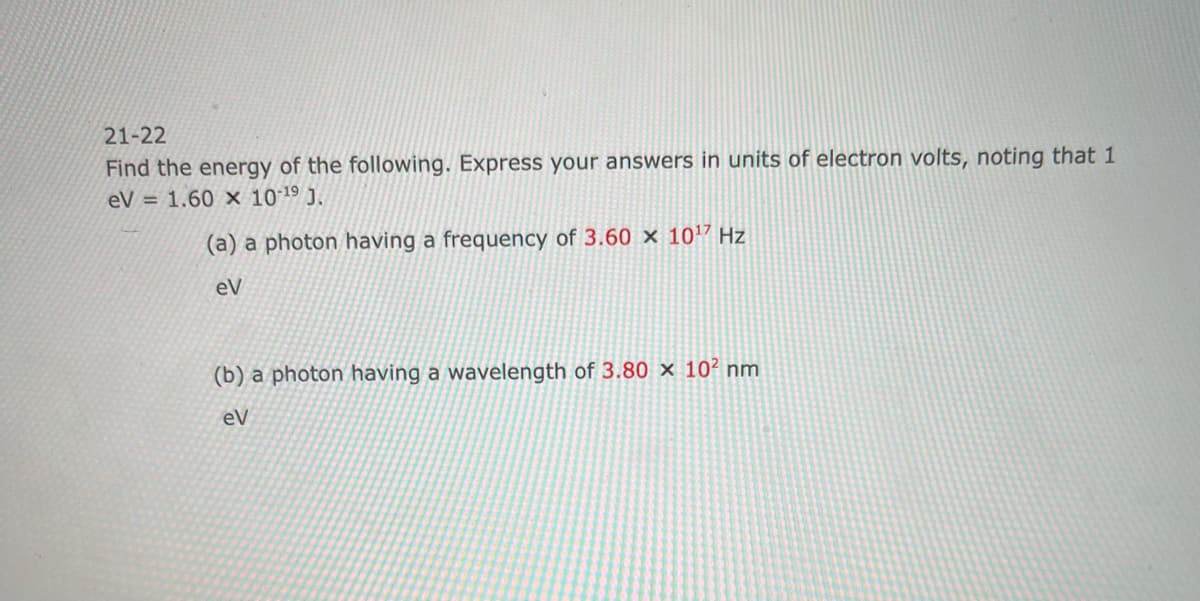
21-22 Find the energy of the following. Express your answers in units of electron volts, noting that 1 eV = 1.60 x 10-19 J. (a) a photon having a frequency of 3.60 x 1017 Hz eV (b) a photon having a wavelength of 3.80 x 102 nm eV
Q: A space probe with a mass of 500kg, circles a planet with a radius of 2580 km radius and a mass of…
A: The objective of this question is to find the acceleration, speed, and period of revolution of a…
Q: The average value of the given voltage is approximately 12.55 V 15 V 10 V Select one: True False
A: For full-wave rectifier: Vavg = 0.637 x VpeakVavg = 0.637 x 15 V = 9.55 V
Q: An empty barrel with a diameter of 1 meter is revolved about its center. Suppose a 1 kg trout is…
A: Step 1:To solve this problem, we can use the principles of rotational motion and the concept of…
Q: ray in Gir makes an normal after leaving 6) A light ray passes, from air thin G plastic slab (n =…
A: basic theory Snell's Law and Refraction Snell's law describes how light bends, or refracts, as it…
Q: Either all parts or none.... Vll upvote
A:
Q: Determine how to calculate the half-life of (see image) from the measurement of its decay rate (or…
A: Based on the information you provided, the image contains text about the element Barium-137 (¹³⁷Ba)…
Q: The wow great expert Hand written solution is not allowed
A: Step 1:Given values are,d=5.02cm±0.02cmt=4.28mm±0.01mm=0.428cm±0.001cmf=11.6cm±0.2cmStep 2:The…
Q: 4.1. Figure 1 shows three points near a very long charged rod. The distance between A and B, the…
A: Step 1: The scenario describes a situation near a long charge rod where the distances between points…
Q: Gg
A: Step 1:Step 2:Step 3:
Q: Two charges Q1 = 2, 3μC and Q₂ = 5, 1μC are placed on the two corners of a right triangle with the…
A: Step 1:Step 2:Step 3:
Q: (a) How high (in m) a hill can a car coast up (engine disengaged) if friction is negligible and its…
A: Step 1:a)Initial speed is 74hkmConversion of speed in terms of meters per…
Q: Needs Complete solution with 100 % accuracy.
A: To find out what happens after the collision, we can use the principle of conservation of momentum,…
Q: In a croquet shot a pair of touching balls are hit sharply by the mallet in the direction of the…
A: Step 1: Before both are in rest , so initial momentum is zero . Step 2: Now the balls are hitted…
Q: I 8:36 AM ← Sam...io 4.7 + 37 K/s 1. An airplane from rest accelerates on a runway at 5.50 m/s² for…
A: Step 1: Step 2: Step 3: Step 4:
Q: Estimate the force per unit length between two parallel wires carrying 0.1 amp of current. Derive…
A: The force per unit length between two parallel wires carrying current can be estimated using the…
Q: = A pendulum consists of a rod of mass 1.80 kg and length L 1.00 m with a solid sphere at one end…
A: Step 1:Step 2:
Q: Needs Complete solution with 100 % accuracy. Don't use chat gpt or ai i definitely upvote you.
A: ----------------------------Solution---------------------------(STEP…
Q: (a) What is the intensity (in W/m²) of a sound that has a level 3.00 dB lower than a 5.17 × 10−9…
A: The objective of the question is to find the intensity of a sound that is 3.00 dB lower and 10.0 dB…
Q: An object of mass 7 kg is given an initial downward velocity of 40 m/sec and then allowed to fall…
A: description and the concepts of thin-film interference, I can still help you with the…
Q: Current Attempt in Progress Redesigning a track. The figure below shows a small block that is…
A:
Q: Needs Complete solution with 100 % accuracy.
A:
Q: At the peak of its trajectory, a 1.0 kg projectile moving horizontally at 15 m/s collides with a 2.0…
A: To solve this problem, we need to calculate the change in momentum of the colliding system and…
Q: Needs Complete solution with 100 % accuracy don't use chat gpt or ai i definitely upvote you. Be…
A: From Fahrenheit to Kelvin (13760°F − 32) × 5/9 + 273.15 = 7899.817K T=7899.817K Now from Wien's law…
Q: Need typed answer and show work
A: Sure, The question states that the position of an object is modeled by a function and asks you to…
Q: Needs Complete solution plz don't use chat gpt or ai i definitely upvote you be careful Downvote for…
A:
Q: Please don't provide handwritten solution.
A: Step 1:
Q: A 0.381 m long metal bar is pulled to the left by an applied force and moves to the left at a…
A: Step 1:Given data:L=0.381mv=5.90m/sB=0.661TR=40.9ΩFrom Faraday's law Induced emf ,V=B∗v∗LStep…
Q: In the figure below, a metal bar sitting on two parallel conducting rails, connected to each other…
A: Part e solution :
Q: A cubical box (equal side lengths) of mass 1.2 kg (outline shown in blue in the figure) is supported…
A: To determine the minimum coefficient of static friction between the cubical box and the horizontal…
Q: Don't use chat gpt
A:
Q: Needs Complete solution with 100 % accuracy don't use chat gpt or ai i definitely upvote you be…
A: The magnitude of the acceleration is 4.472 m/s^2, and the direction is 333.435° from the positive…
Q: The hand written solution is not allowed please
A: key concept1. find work done; we need to solve integrals. In solving the integral we repleced some…
Q: None
A:
Q: A uniform solid sphere rolls down an incline. (a) What must be the incline angle (deg) if the linear…
A:
Q: 3. The acceleration of a particle is directly proportional to the square of the time t. When t = 0,…
A: Step 1:Step 2:Step 3:Hope this helps!
Q: A total lunar eclipse is observed on December 31. Predict the next lunar eclipse. A total lunar…
A:
Q: mi A cart of mass m₁ = 10 kg slides down a frictionless ramp and is made to collide with a second…
A: Step 1:
Q: (Figure 1) shows a 12-cm-diameter loop in three different magnetic fields. The loop's resistance is…
A: Step 1:Step 2:Step 3:Step 4:
Q: Anil
A:
Q: Suppose a vertical tunnel () is dug along the diameter of the earth assuming a sphere of uniform…
A: Step 1:Mass of the body is mDensity is ρStep 2:The formula for the acceleration will…
Q: The wow expert Hand written solution is not allowed
A: Step 1: Step 2:
Q: Billiard ball A and billiard ball B are each moving at speed & towards the other. After their…
A:
Q: Two particles are in a uniform electric field whose value is +2500 N/C. The mass and charge of…
A:
Q: A block of mass m = 3.00 kg situated on a rough incline at an angle of 0 = 37.0° is connected to a…
A:
Q: A concave lens forms a virtual image 0.5 times the size of the object. The distance between object…
A:
Q: 6) Calculate the electric field over point P generated by a linear rod of length L. and charge…
A:
Q: I keep getting 2.23e-6
A: The electric potential energy lost by a system.Analysing the problemThe system consists of a proton…
Q: Two baseballs of diameter 7.35 cm are connected to a rod 7 mm in diameter and 56 cm long, as in the…
A: Step 1:Given that, Rod diameter (d) = 7mm Rod length (L) = 56 cm speed (N) = 340r/min N = 340 rpm…
Q: Using the image that demonstrates a binding energy per nucleon graph, explain why the heaviest of…
A: Step 1:Step 2:
Q: Can you please go in depth and break this problem down step by step because I'm really struggling.…
A: Sure, The problem asks you to find the force on a magnetic dipole placed at the origin due to a…
Step by step
Solved in 2 steps
