
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
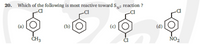
Transcribed Image Text:20. Which of the following is most reactive toward Sy2 reaction ?
Cl
-Cl
Cl
(a)
(Ъ)
(c)
(d)
ČH3
NO2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 7 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the physical states for the products and then determine which factor does NOT drive reactions (i) and (ii) to product formation? 1) 2KO2(s) + 2H2O(1) → 2KOH(?)+H2O(1) + O2(?) (ii) 4KO2(s) + 2CO2(g)→ 2KCO3(?)+302(?) Select one: O A. For (i), formation of a water O B. For (ii), formation of O2 O C. For (ii), formation of K2CO3 O D. For (i), formation of a gas O E. For (i) and (ii), generation of a more stable oxidation state for Oarrow_forwardWhich of the following molecules is least acidic? A OE O B O O C A B C D Earrow_forwardname both compounds. consider steeochemistry. Please help with some explanation.arrow_forward
- Show all the bonds in the drawing please dont do ch3ch2ch draw it out tyarrow_forward12arrow_forwardinvolvedinthereaction? 4.Theenthalpyforthereactionbelowis-25kJ: 2A-A+B=B→2A-B-A a.Giventhefollowingbondenergies,findthemissingvalue. b. Sketch a potential energy diagram for this reaction:arrow_forward
- What is the enthapy of the reaction of C2H4O + H2 ---> C2H6O in kJ a. -484kJ b. -366kJ c. -48kJ d. 48 kJ e. 366 kJ Please see attached image for structure and bond energies given in problemarrow_forwardWhich of the following reactions most favors products? REACTION 2 SO22) +O2(g) 2 2s0:(2) 2.6 x 102 2 NO) +O2e) 2NO%) 6.4x105 II 2C0e) +02(e) 2 2COe) 2 2002(g) 2.5x 1015 III 2 H2(8) + O26«) 2 2H2Og) 1.7x1027 IV а. I b. II С. II d. IV |3arrow_forwardPls help ASAP.arrow_forward
- Find the AHrn for the following reaction: 2N29) + 502(9) → 2N,O5(9) given the following reactions and subsequent AH° values 2H2(9) + O2(9) → 2H,Ou) N½O5(9) + H2Ou → 2HNO3(1) AH° = -541.6 kJ AH° = -54.6 kJ %D N2(9) + 302(9) + H2(9) → 2HNO3(1) AH° -331.1 kJarrow_forwardHow many of the processes below have a positive AS? H;O() - H;O(g) The heating of 35 g of water from 99°C to 100°C Cu()- Cu(s) 2 NH(g) - Nalg) + 3 Ha(g) OAO 084 OC1 OD.2 OE3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY