
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
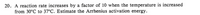
Transcribed Image Text:20. A reaction rate increases by a factor of 10 when the temperature is increased
from 30°C to 37°C. Estimate the Arrhenius activation energy.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Show me the balances for each subsequent system: overall, air conditioner, mixing point, and splitting point. Material balancesarrow_forwardPrepare a material flowsheet similar to Figure 6.1 (shown below) for production of 2400 lb-mol/hr of Vinyl Chloride (VC) based on the reaction path #5 with the operating parameters provided below. You MUST show the Molar flow rates of ALL compounds in ALL streams in your flowsheet. Reaction Path #5. Balanced process for Chlorination of Ethylene C2H4 + Cl2--> C2H4CI2 (C) Ileat Liberated Ileat Absorbed y Reactinn 150 x 10 Blutr duriag Reaction = 52 x 10 Bluhr C2H4 + 2HCI + 1/2 O2--> C2H4CI2 + H2O 2C2H4CI2 --> 2C;H3CI + 2HCI (E) (D) 58.300 Ibhr 2C2H4 + Cl2 +1/2 O2 --> 2C2H3CI + H2O (C) 100% Conv. of Cl2 at 80°C and 150 kPa with 15% (G) Direct Chlorination YƯ C, 15 atm Pyrolysis Sorc +CH,CI- 26 ata HCI CH.CI, CAL 11.3,4KI hr 158,I Ihyhr CH,CI, C1,CI 4,900 Ihhr CH, -a CH,CI, excess C2H4 CH,ClCH,CI + HCI (E) 100% Conv. of HCl at 275°C and 150 kPa with 5% 105,500 Ib'hr excess C2Н4 (D) 75% Conv. At 500 °C and 3000 kPa. Water is separated from the mixture before entering Figure 6.1 reactor D.arrow_forwardEnglish: "Application example for reaction: Carbon oxidation The abbreviation TOC stands for Total Organic Carbon, representing the total carbon content of a thermally treated waste sample. In this case, the decision needs to be made whether the residues from thermal waste treatment can be deposited in a landfill. For this purpose, the criterion TOC ≤ 3 wt-% must be met. Calculate the carbon content of the thermally treated waste sample and check if the criterion is fulfilled. Given: 10 g thermally treated waste sample • A total of 11.207 standard liters of gas is formed during TOC analysis The gas has a CO2 content of 4 mol-% • The resulting gas can be considered as an ideal gas."arrow_forward
- Demonstrate the atomic species balances for the answers, and demonstrate the molecular species balances for the answers. Do not use the extent of reaction.arrow_forwardGIVE A SIMPLE CALCULATION ON HOW TO GET TO 300LA first order irreversible reaction is conducted in an 800 L continuously stirred tank, in liquidsolution at 80 ᵒC temperature. The total conversion in the tank is calculated to be 60%. However,this conversion appears low.After a quick check, you realise that something in the mixing is not working correctly and as aresult, the tank is not optimally stirred.Following a few tests on residence times, you calculate that approximately 300 L of the tank do notinteract appreciably with the input or output streams. You therefore decide to modify the reactorby improving the stirring performance in order to obtain complete mixing in the reactor volume.arrow_forwardThe rate constant k for a certain reaction is measured at two different temperatures: temperature k 325.0 °C |1.7 × 1012 456.0 °C |3.0 × 1013 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E, for this reaction. Round your answer to 2 significant digits. kJ E = Ox10 molarrow_forward
- please quickly thanks !!!!arrow_forwardA binary distillation is performed so that a distillate 94% and bottoms 8% in of the more volatile Component A results. The process shown below reflects one operating condition of a feed stream. Rectification, Stripping and the q-line are all provided on the x-y diagram below. Aling with expected stage for the distillation.. What is temperature of the liquid stream leaving the reboiler? What is the temperature of the vapor stream leaving the feed stage? What is the temperature of the liquid stream leaving stage 2? What is the temperature of the liquid reflux returned to the column? Note: A total condensor is installed.arrow_forwardExamine the reaction equation below. "Heat" being present on the reactant side of an equation indicates what? "Heat" + 2NH3 N2 + 3H2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The