
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
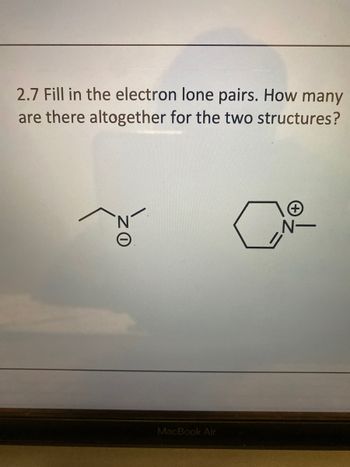
Transcribed Image Text:2.7 Fill in the electron lone pairs. How many
are there altogether for the two structures?
ZO
MacBook Air
N-
Expert Solution
arrow_forward
Step 1
Lewis’s structure: Lewis’s bonding theory is based on the octet rule. The Lewis structure is a simplified representation of the valance shell electrons (bond pair and lone pair) of a molecule.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- do all pleasearrow_forwardIdk itarrow_forward(11) Try Again Your answer is incorrect. • BP: The name of this compound is not "boron phosphide". Be sure you know the correct names of all elements in this compound. Check the Periodic Table if necessary. • C3S2: Your answer is incorrect. Fill in the systematic names of the following chemical compounds. Note: for compounds containing hydrogen, you may give the common name instead. molecular formula BP C₂S₂ CS₂ BI₂ H₂O Explanation Recheck name of compound boron phosphide carbon subsulfide carbon disulphide boron triiodide water X Ś olo Ararrow_forward
- 2.) A molecule of XeF4 has x bond pairs and y lone pairs around the central atom. Write your answer as x = # and y = #. Make sure there is a space between characters.For example, x = 2 and y = 5.arrow_forward5. Which of the following statements is incorrect? A. AICI3, Ba(NO3)2, and CuSO4 are examples of ionic compounds. lonic solids dissolved in water dissociate to form ions В. C. lonic bonds are formed between metals and nonmetals. D. When forming ionic bonds, metals and nonmetals share electrons.arrow_forwardGive two examples of elements that are able to form stable compounds with fewer than 8 electrons around the central atom?arrow_forward
- 5. Fill in the empty boxes with the correct information. 6. 1st Element Ag # of atoms 1 b. Ionic bond: 2nd Element c. Polyatomic Ion NO₂ # of atoms Give three chemical formulas and corresponding names for each bonding pattern: a. Covalent bond: 81 Formula C8H10 Name Ammonium phosphate Molybdenum (II) acetate Potassium telluratearrow_forwardHow can I tell the charge of NaCH3COO, NaCl, and HCH3COO? Just in general I am not sure of how to determine the charge of compounds, so if that could be explained?arrow_forward3. By means of Lewis structures, represent bonding between the following pairs of elements (Your structures should show whether the bonding is essentially ionic or covalent): (2 pts each) (d) Cs and Cl (e) Li and 0 (f) Cl and Iarrow_forward
- Determine the number of atoms in the term 12Al2(SO4)3? Also, what does the 12 in thr front of the formula signfy?arrow_forwardGiven the following (fictional) ions: cations anions D+1 M1 E+2 (LB4) 2 (ZG2)*3 (ZEm) 2 At3 R 3 Write the balanced chemical formula for the ionic compound that would form between the following pairs of ions. a) ZG2 and D*1 b) A and LB4arrow_forwardPayalbenarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY