
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
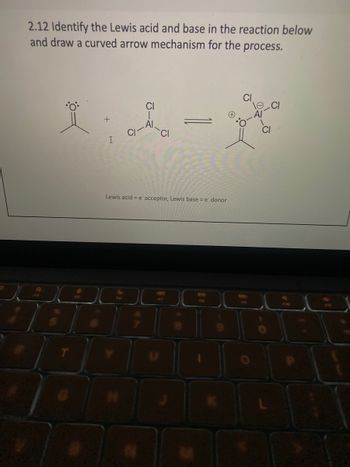
Transcribed Image Text:2.12 Identify the Lewis acid and base in the reaction below
and draw a curved arrow mechanism for the process.
I
I
CI
CI
CI
Lewis acid = e acceptor, Lewis base = e donor
20
DC
990
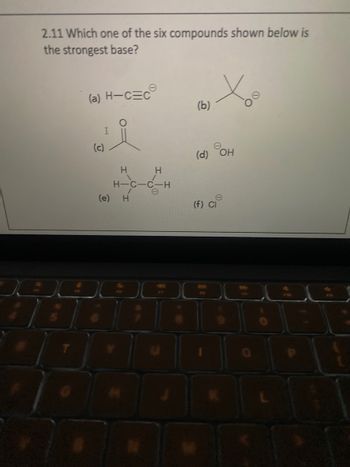
Transcribed Image Text:2.11 Which one of the six compounds shown below is
the strongest base?
(a) H-CEC
(c)
I
(e)
H
H
H-C-C-H
H
80
(b)
(d) OH
(f) Cl
شات
F10
6
Expert Solution
arrow_forward
Step 1
Since you have asked multiple type questions, we will solve only first questions for you. If you want any specific question to be solved then please specify the question number or post only that question.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- - 30 2 W 3C₂²- 1 Write the formula of the conjugate acid of the Brønsted- Lowry base, PH3 + for additional resources 3 2 (s) $ 4 3 H % 5 Reset 4 (1) Question 15 of 28 6 5 ) & 7 6 (g) P 8 7 个 8 • x H₂O 9 ) 0 9 (aq) Oarrow_forwardCIRCLE AND LABEL THE LEWIS ACID AND LEWIS BASE SITES FOR THE FOLLOWING COMPOUNDS: ΝΗ Ï Y DRAW RESONANCE FORMS FOR THE FOLLOWING ΝΗ Ï Harrow_forwardWhich corresponds to the best answer?arrow_forward
- Malic acid (H,CHO) derives its name from the genus name for apples (Malus), from which it was first isolated. It is the main acid in many fruits (including apricots, cherries, peaches and pears) and is widely used as a food additive to enhance sweetness or tartness. Malic acid is a diprotic acid with pK 3.40 and pK, = 5.11 at 25°C. !3! al Calculate the following for a o.20 M malic acid solution at 25°C. pH= %3D [HC,H 0,]= [HC,H,O,1=arrow_forward3.27 For each pair of structures below, identify the stronger acid, and explain your choice: H H N=H -N H H H (a) (c) H -" :0 H ΝΞΗ I H ΗΘΗ `N' H CONTA ΘΝ H (b) (d) H H H N H I Harrow_forwardG.128.arrow_forward
- For each of the molecules shown below, draw the acid dissociation reaction. If a conjugate base has resonance structures, draw all valid resonance structures. AH B C ہو A & B H B & C CF3 A & C H+ + Identify the stronger acid in each of the pairs below; provide an explanation for each pair. H+ + H+ +arrow_forwardSeeimsge belowarrow_forwardPlease assist the student with the following problems they are struggling witharrow_forward
- Unanswered •1 attempt left • Due on Mar 12 Which of the following compound could NOT act as a Lewis base? H2O OH^- NH3 D NH4^+ НFarrow_forwardNonearrow_forward2- 3C₂² 1 Write the acidic equilibrium equation for CCI₂HCOOH. Be sure to include the proper phases for all species within the reaction. + 2 (s) H₂O ap here or pull up for additional resources 3 H ( Reset 4 (1) Question 62 of 75 O LO 5 ) 6 x H₂O CI 7 (g) 8 OH 9 12 (aq) C X 0 LTE 23% Submitarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY