2.1 Determine the wavelength of photon emitted when an electron moves from n = 2 orbit to n = 1 orbit in a gold atom. If Z is the atomic number, and for gold Z = 79. Also, by how much energy will the bombarding electrons excite the gold atom to radiate this emission line?
Q: Which of the following is not a prediction made by Einstein's Theories of Relativity? Select one:...
A: Option D is correct.
Q: An object with mass 0.30 kg falls from rest onto a spring of stiffness 25,000 N/m. The relaxed heigh...
A:
Q: Two cars are travelling east along a straight highway at the same speed. At an intersection, the hig...
A: Given: Two cars are travelling east along a straight highway at the same speed. The angle between th...
Q: Spherical waves that are emitted from a point source; generate the disturbance Φ = 4.2 cos(600 t+ 60...
A:
Q: An antique coin is a mixture of silver and copper. The coin has a mass of 1.120 10-2 kg and in water...
A:
Q: A force of 8 pounds stretches a spring 1 foot. A mass weighing 3.2 pounds is attached to the spring,...
A:
Q: An Atwood's machine consists of blocks of masses m1 = 11.0 kg and m2 = 22.0 kg attached by a cord ru...
A: (a) Since the pulley is rotating in the clockwise direction, there must be a non-zero torque on it. ...
Q: Suppose a person wishes to lift a large rock with a lever. If the rock has a mass of 250kg and is lo...
A: Given, mass of rock = 250 kg
Q: How is the obtained spectrum from fluorescent light, LED light, incandescent light, candle light and...
A: Fluorescent light spectrum: Fluorescent light sources emit visible light when an electric charge is ...
Q: A horizontal disk rotates about a vertical axis through its center. Point P is midway between the ...
A:
Q: The two blocks (MA = 100 kg, MB = 150 kg) shown are originally at rest. Assuming that the coefficien...
A: (a) Consider the following figure for the motion of block A,
Q: Advanced Physics Question
A: Using the formula of inductance
Q: An object is subjected to a friction force with magnitude 4.63 N. which acts against the object's ve...
A: (a) Work done to move an object from O to A along purple path and then return to O via purple path W...
Q: The circuit in the figure consists of switch S, a 4.50 V ideal battery, a 35.0 M2 resistor, and an a...
A:
Q: Suppose that a moderately-sized house has inside dimensions 15mx25mx2.4m, and that all air is replac...
A: Heat transfer per unit time Qt=mcΔTt=ρVcΔTt=ρlbhcΔTt
Q: A block of mass m is moving with speed v along a horizontal surface when it collides with a uniform ...
A: Hello. Since you have posted multiple questions and not specified which question needs to be solved,...
Q: Please very soon
A:
Q: What is the total distance? What is the average speed of this object? What is the displacement? What...
A:
Q: Po-204 (mass=204 amu) undergoes alpha decay. Show that, as a result of ejecting alpha particle (He-4...
A: Let m denotes the mass of Po-204 atom, m1 and m2 denote the alpha particle and daughter particle mas...
Q: When ultraviolet light with a wavelength of 400 nm falls on a certain metal surface, the maximum kin...
A: Using photoelectric formula,
Q: Once fusion stops in the core of a star, the core is primarily supported against gravitational colla...
A: Answer: As per the above-given data, Once fusion stops in the core of a star, the core is prima...
Q: 4. m = 5×10-° Kg is %3D a) A point charge for which O = 2 × 10-l°C and E = 100ā, – 200a, + 300a, y/m...
A:
Q: NASA launches a rocket at t=0t=0 seconds. Its height, in meters above sea-level, as a function of ti...
A:
Q: Say I want a transformer to step up voltage from 120 V to 300 V. If the primary coil has 1000 turns...
A: Given, primary voltage = 120V secondary voltage = 300 V and coil turn in primary = 1000
Q: A spaceship with a length of 300 meters is in your hand at 0.750 us from the front of the observer o...
A:
Q: Consider a resistor and an inductor is connected in series with a battery. Drive an expression for e...
A:
Q: 12. Take two ideal polaroid (the first with its axis vertical and the second , horizontal) and inser...
A: Given: The Two Polaroid with stack of 10 half wave plates inserted between them with the axis rotati...
Q: Question number 12 & 13
A: “Since you have asked multiple question, we will solve the first question for you. If you want any s...
Q: Find the particle's horizontal position x(t) and velocity v(x) at any point in a fluid whose drag fo...
A: Part (a) Given: The drag force in the particle is Fdrag=kmv. The formula to calculate the net forc...
Q: A 69.0-kg person throws a 0.0495-kg snowball forward with a ground speed of 32.0 m/s. A second perso...
A: Given: Mass of thrower= 69 kg Mass of snowball=0.0495 kg Group speed=32 m/s Mass of catcher=59.5 kg ...
Q: A horizontal 1-m-square plate electric heater dissipates heat to the atmospheric air at 25oC. The po...
A: Given thatTsurface=85°CTatmosphere=25°CPower of electric heater,Q= 0.5 kwQ=500wattas we know Q=hA(Ts...
Q: Advanced Physics Question
A:
Q: Carbon tetrachloride at 600F (density is 99.5 lb/ft3 and viscosity is 8.064 x 10-4 lb/ft-s) is flowi...
A: Given that : Density = 99.5 lb / ft3 Viscosity = 8.064 * 10-4 lb/ft Carbon tetrachloride at 600F I...
Q: How does the work required to accelerate a particle from 10 m/s to 20 m/s compare to that required t...
A: Kinetic energy is associated with every body in motion as KE=.5mv2 ⇒KEαv2 In first case, KE=0.5m(v22...
Q: Josh is painting yellow stripes on a road using a paint roller. To roll the paint roller along the r...
A: Moment of Inertia The moment of inertia of a rigid body is equal to the sum of the product of the ma...
Q: P1 A pendulum of length L with a bob of mass m is released from the vertical position without initia...
A: Let L denotes the thread’s length, x denotes the distance of the nail from the bob (radius of the ci...
Q: Once carbon builds up in the Sun's core, astronomers expect our Sun to first become a red giant, the...
A: Our sun is an average mass star in the main sequence. The life cycle of average main sequence star i...
Q: A ship headed due east is moving through the water at a constant speed of 12 miles per hour. However...
A:
Q: The radius of a certain segment of pipe is reduced to 85% of its original value due to buildup. At t...
A: Given information: The radius of the segment of the pipe is reduced by 85 % of its original value. r...
Q: V0=12 m/s S0= 300 m a1=8 m/s2 a2=0 a3=-3 m/s2
A: Given And at t=0 V0=12 m/s S0= 300 m Now we know With V=velocity at any instant T, and Vi=velocity...
Q: Advanced Physics Question
A: Correct options:- (1) (X=Z) > Y (2) Option (E)- All three-sphere collide with the same speed. Exp...
Q: Suppose that you have three vectors: f1 (x) = 1, f2 (x) = x – 1, and f3 (x) = } (x² – 4x + 2), that ...
A: The inner product is given by Thus for derivative operator D The standard integral gives ...
Q: 1) A cart having mass mc is connected to a hanging mass mp as shown in the figure. When the cart is ...
A:
Q: Show that the kinetic energy of an object rotating about a fixed axis with angular momentum L = Iω c...
A: Solution: Given Statement: To show that the kinetic energy of an object rotating about a fixed axi...
Q: As shown in the figure below, current is flowing along the corners of the cube. Obtain the magnetic ...
A: Given that : Let us consider the current is flown around the edges of the cube. The magnetic field s...
2.1 Determine the wavelength of photon emitted when an electron moves from n = 2 orbit to n = 1 orbit in a gold atom. If Z is the atomic number, and for gold Z = 79. Also, by how much energy will the bombarding electrons excite the gold atom to radiate this emission line?
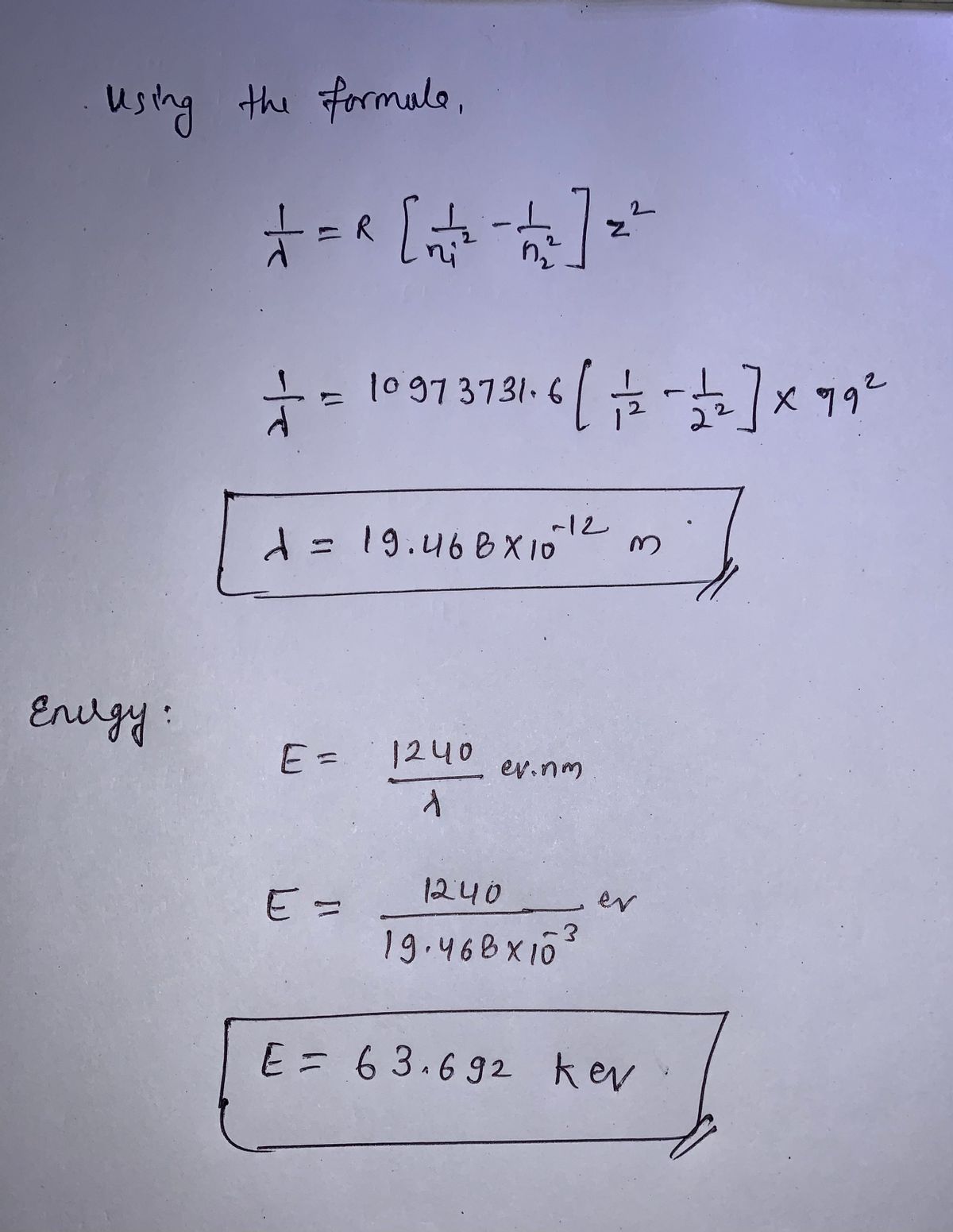
Step by step
Solved in 2 steps with 1 images
