
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
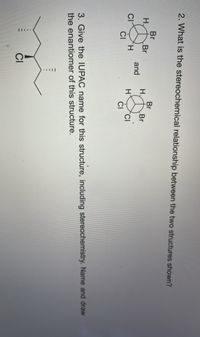
Transcribed Image Text:2. What is the stereochemical relationship between the two structures shown?
Br
Br
H.
Br
H.
Br
and
CIYH
CI
CI
3. Give the IUPAC name for this structure, including stereochemistry. Name and draw
the enantiomer of this structure.
CI
Expert Solution
arrow_forward
Step 1- Introduction
Stereoisomerism is the kind of isomerism, in which molecules have same molecular formula, but different arrangements of the bonds in the three dimensional space. The resulting molecules are known as stereoisomers. These are of various kinds. Rotation around single bonds are possible. So, there can be as many stereoisomers as many rotations are possible. While rotating, molecules acquire the geometries in which they are most stable and most unstable. A constant interchange between these structures keeps on happening.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the correct Lewis structure for propanoic acid, a carboxylic acid which has the molecular formula C3H,O,? H H :0: H H H :0: H H H H H :0: H. H FC 0 -H H FC H FC H H- H H H a b e Oa. Ob. Oc. Od. Oe.arrow_forwardAbout t You dor esc L ! 1 compound Q A etches: the lines stand for chemical bonds between the atoms. Just ignore the dots. They stand for "lone pairs, and you'll learn about the ed to know anything about lone pairs to solve this problem. N A B с D Explanation 2 W S 28 command X H-O-H H-O-C- H :O: || H-C-C-Ö * sketch of molecules in it Check Н # 3 HO: H :O: H H. E D ī H -0-0-H 4-0- 20 -0- C Н H :0: C C $ 4 C :O: ö HÖC=C-Ö-H -C-0-H H R H :0: 0: F 888 :0: % 8 5 V HIGIA T G I -H A 6 group (Choose one) ▼ (Choose one) ▼ (Choose one) ▼ B (Choose one) ▼ MacBook Pro Y H & 7 4 U N © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | * 00 X ▶II 8 J - M ( 9 K O V K -0 O L 4 P command - A {arrow_forwardAcetic acid (CH3CO₂H) dissolved in water is shown to the right. Which of the labeled interactions represent hydrogen bonding interactions? H I 0: Oll only H OI only O III only I, III, and IV I and III O I and IV III and IV 4. HICH III 0: -C- &mm. :O: II -H H ||||.* H IV Harrow_forward
- 14. The carbon with the largest delta value (the most deshielded) is يعلمهم ЊС a. a b b. b C. Carrow_forwardPlease help me complete thisarrow_forwardWhich of these pairs are resonance structures? i. ii. 道. H H-C H H H H-C–C–C—C—H H H H H H | H H 一 H H H–C–C H H-C H H H H C H 大学 6: H :OH O:H H:O: H H :Ö: H C-H OH :0₁arrow_forward
- L A Moving to another question will save this response. Question 6 For collisions to be successful, reactants must have O a. favorable geometry only ☐ b. sufficient heat of reaction only ☐ c. sufficient kinetic energy and favourable geometry ☐ d. sufficient potential energy only A Moving to another question will save this response. SOLarrow_forwardN. H3O+ Qarrow_forwardWhat is the correct Lewis structure for acetaldehyde, an aldehyde which has the molecular formula CH,O? H :0 :0 H- H :0: H :0: H -č: FC-H H- -H H- -C-H H- C -ċ-H H- H H H a b d e a. Ob. Oc. Od. Oe.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY