
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Hi can you please help with with questions 2 and 8. Thank you so much!
2. What is the molecular formula of your mononitrated reaction product?
8. Based on all of the data provided, what is the identity of the “G” group? Please NEATLY & CLEARLY draw its full Lewis structure in the space provided
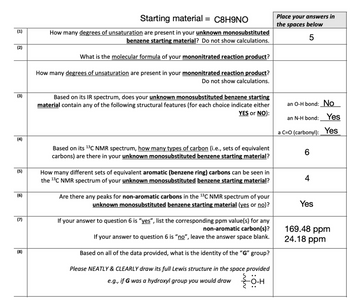
Transcribed Image Text:(1)
(2)
(3)
(4)
(5)
(6)
(7)
(8)
Starting material = C8H9NO
How many degrees of unsaturation are present in your unknown monosubstituted
benzene starting material? Do not show calculations.
What is the molecular formula of your mononitrated reaction product?
How many degrees of unsaturation are present in your mononitrated reaction product?
Do not show calculations.
Based on its IR spectrum, does your unknown monosubstituted benzene starting
material contain any of the following structural features (for each choice indicate either
YES or NO):
Based on its ¹³C NMR spectrum, how many types of carbon (i.e., sets of equivalent
carbons) are there in your unknown monosubstituted benzene starting material?
How many different sets of equivalent aromatic (benzene ring) carbons can be seen in
the ¹3C NMR spectrum of your unknown monosubstituted benzene starting material?
Are there any peaks for non-aromatic carbons in the ¹3C NMR spectrum of your
unknown monosubstituted benzene starting material (yes or no)?
If your answer to question 6 is "yes", list the corresponding ppm value(s) for any
non-aromatic carbon(s)?
If your answer to question 6 is "no", leave the ans
ver space blank.
Based on all of the data provided, what is the identity of the "G" group?
Please NEATLY & CLEARLY draw its full Lewis structure in the space provided
e.g., if G was a hydroxyl group you would draw {0-H
Place your answers in
the spaces below
5
an O-H bond: No
an N-H bond: Yes
a C=O (carbonyl): Yes
6
4
Yes
169.48 ppm
24.18 ppm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please help solve allarrow_forwardTwo major resonance structures are possible for the anion shown. One resonance form is given, but it is incomplete. Complete the given structure by adding nonbonding electrons and formal charges. Draw the remaining structure, including nonbonding electrons and formal charges. Omit curved arrows. Structure A: complete the structure by adding nonbonding electrons and formal charges. H H H I Structure B: draw the remaining resonance structure, including nonbonding electrons and formal charges. H- : z: H Harrow_forwardIf both could be answered that would be great!arrow_forward
- Help me answer number 10 pleasearrow_forwardSide by side dot structures of two water moleculs are given below. Draw structures like that on your paper. Put partial positive and partial negative charges where appropriate. Show two hydrogen bonds by connecting the partial positive charge on one molecule to the partial negaive charge on the other molecule using a dotted line. Upload your answer here. H-O-H H-O-Harrow_forwardDirections: Read the following statements. Write your answer in a separate sheet of paper. Lewis dot ion after electron symbol of each ion if ionic bond is formed Charge of each Lewis dot Туре of Bond Atoms symbol of each atom Formula of the transfer if ionic involved Product bond is formed Na, O Са, N S, CIarrow_forward
- Consider the following incomplete structures. On your rough sheet, complete their correct Lewis structures (including minimizing formal charge) by adding electrons/bonds where necessary. Which statement below is true of the completed Lewis structures? Hint: Consider the potential for multiple bonds on the molecules and be sure to account for all valence electrons. The NOCI," exhibits both formal charges and resonance hybrids, while the BrOCI," exhibits resonance hybrids but no formal chargés None of these. The NOCI, exhibits formal charges but no resonance hybrids, while the BrOCI," exhibits both formal charges and resonance hybrids The NOCI, exhibits both residual formal charges and resonance hybrids, while the BrOCI, exhibits formal charges but no resonance hybrids At least one atom in each molecule exhibits formal charges, and the molecules have no resonance hybrids The BrOCI, exhibits formal charges but no resonance hybrids, while the NOCI, exhibits resonance hybrids but no formal…arrow_forwardFor the given compound, draw all significant resonance forms and rank them from most significant to least significant. Briefly explain the rankings. Part 1 Let's begin by considering which resonance patterns are present. First, add curved arrow(s) to show the resonance using the following pattern: a pi bond between two atoms of differing electronegativity. Modify the second structure given to draw the new resonance structure. Include relevant formal charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds. H₂C CH₂ H₂C Edit Drawing CH₂ SUPPORTarrow_forwardplease help solve allarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY