
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
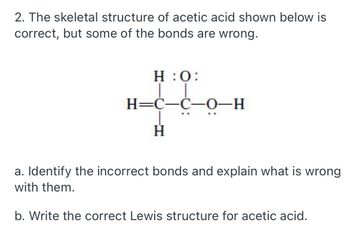
Transcribed Image Text:2. The skeletal structure of acetic acid shown below is
correct, but some of the bonds are wrong.
H:O:
co
H=C-C-0-H
H
a. Identify the incorrect bonds and explain what is wrong
with them.
b. Write the correct Lewis structure for acetic acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How does the bond energy of HCl(g) differ from the standard enthalpy of formation of HCl(g)?arrow_forwardConsider the pyrosulfate ion, S2O72-. It has no sulfur–sulfur nor oxygen–oxygen bonds. (a) Write a Lewis structure for the pyrosulfate ion using only single bonds. (b) What is the formal charge on the sulfur atoms for the Lewis structure you drew in part (a)? (c) Write another Lewis structure using six bonds and two O—S bonds. (d) What is the formal charge on each atom for the structure you drew in part (c)?arrow_forwardA common trait of simple organic compounds is to have Lewis structures where all atoms have a formal charge of zero. Consider the following incomplete Lewis structure for an organic compound called methyl cyanoacrylate, the main ingredient in Super Glue. Draw a complete Lewis structure for methyl cyanoacrylate in which all atoms have a formal charge of zero.arrow_forward
- hat does temperature measure? Are the molecules in a beaker of warm water moving at the same speed as the molecules in a beaker of cold water? Explain? What is heat? Is heat the same as temperature?arrow_forwardMethanol, H3COH, is used as the fuel in some race cars. Ethanol, C2H5OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO2 and H2O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.arrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D chemical symbol, chemical formula or Lewis structure .. 0=N-C1: : Cl : : Cl: CaCl₂2 .. Cl₂ C=C : Cl: : Cl: boiling point (Choose one) (Choose one) v (Choose one) ✓ (Choose one) ✓ X 5arrow_forward
- 4. The following are valid Lewis structures for CH3SOCH3. Label the formal charges and circle the best Lewis structure. H :ö: H H :0 H a) H-C=S-C-H b) Н—С—S— С-н H. H. H. H. Н :0: Н H :ö: H d) H-C=$-C-H c) Н—С—$—С-н H. H. H Harrow_forwardР ed Lewis structure H-CC-H [H_H_O_H]* Η N_0_a: Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:*arrow_forwardH 2 H. + ::H The Lewis representation above depicts a reaction between hydrogen (blue) and a main-group element from group в (red). In this representation, each Y atom needs bond(s) with atoms of H. electron(s) to complete its octet, and gains these electrons by forming There are unshared electron pair(s) and bonding electron pair(s) in the product molecule. The bonds in the product are Submit Answer Retry Entire Grouparrow_forward
- 1. What is the relationship between the following compounds? H :0: H H:0: H H-C=C-C-C-C-H H-C-C-C=Ċ-H H HH H. H-C-H B. constitutional isomers C. the same structure A. isotopes D. composed of different elements E. no relationship 2. What is the correct Lewis structure for HN3, including the formal charges? CIHarrow_forward4arrow_forwardChoose the best Lewis structure for CH2C12. H. a. H. 6. H–Cl–C-Cl-H H-H-C-Cl-Cl: H. Cl=c=Cl: d. Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning