
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
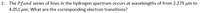
Transcribed Image Text:2. The Pfund series of lines in the hydrogen spectrum occurs at wavelengths of from 2.279 µm to
4.051 µm. What are the corresponding electron transitions?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A ground state hydrogen atom absorbs a photon of light having a wavelength of 92.3 nm. What is the final state of the hydrogen atom?arrow_forwardWhen metallic sodium is dissolved in liquid sodium chloride, electrons are released into the liquid. These dissolvedelectrons absorb light with a wavelength near 800 nm.Suppose we treat the positive ions surrounding an electroncrudely as defining a three-dimensional cubic box of edgeL, and we assume that the absorbed light excites the electron from its ground state to the first excited state. Calculate the edge length L in this simple model.arrow_forwardThe first line of the Balmer series occurs at a wavelength of 656.3 nm. What is the energy difference between the two energy levels involved in the emission that results in this spectral line?arrow_forward
- A bright violet line occurs at 435.8 nvm in the emission spectrum of mercury vapor. What amount of energy, must be released by an electron in a mercury atom to produce a photon of this light?arrow_forwardPlease answer both parts with small explanation or leave for someone elsearrow_forwardCalculate the longest and shortest wavelengths of the highest and lowest energy of the Lyman emission line of hydrogen. 1. What is the angular momentum in the lowest energy state of the Bohr hydrogen atom? 2. What is the velocity of the electron in the lowest energy state?arrow_forward
- The line of longest wavelength in visible light for the emission spectrum of hydrogen, 656nm (Balmer series), would correspond to what electronic transition? Edit View Insert Format Tools Table 12pt v Paragraph v I U A ts 区 Warrow_forwardOne of the lines in the spectrum for the boron ion has a wavelength of 518.0 nm. Calculate the energy of the wavelength.arrow_forwardWhy is it significant that colour emitted from the hydrogen emission spectrum is not whitearrow_forward
- If you double the concentration of the hydrogen in the hydrogen lamp, would you expect the wavelengths of the observed lines to double/be cut in half/stay unchanged? Explain.arrow_forwardWhich statement below is true with regard * ?to Bohr's model of the atom The model accounted for the absorption spectra of atoms but not for the emission spectra. The model could account for the emission spectrum of ydrogen and for the Rydberg equation. The model was generally successful for all atoms to which it was applied. The model was based on the wave properties of the electron. 0 هذا السؤال مطلوبarrow_forwardA set of photons have energy 21.8 kJ mol-1. What wavelength is associated with the photons?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY