
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
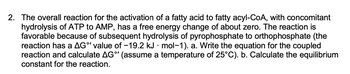
Transcribed Image Text:2. The overall reaction for the activation of a fatty acid to fatty acyl-CoA, with concomitant
hydrolysis of ATP to AMP, has a free energy change of about zero. The reaction is
favorable because of subsequent hydrolysis of pyrophosphate to orthophosphate (the
reaction has a AG°' value of -19.2 kJ · mol-1). a. Write the equation for the coupled
reaction and calculate AGº' (assume a temperature of 25°C). b. Calculate the equilibrium
constant for the reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- At what substrate concentration would an enzyme with a kcat of 33.0 s-1 and a Km of 0.0046 M operate at one-tenth of its maximum rate? Assume the enzyme obeys Michaeli s-Menten kinetics.arrow_forwardGiven that the AG" values for the hydrolysis of glucose 1-phosphate and glucose 6-phosphate are approximately -21 kJ/mol and –14 kJ/mol, respectively, which statement is true regarding the isomerization shown? glucose 1-phosphate → glucose 6-phosphate O The reaction can likely proceed in either direction and will therefore depend on the concentrations of glucose 1-phosphate and glucose 6-phosphate. O Only the anabolic direction will occur because the free energy change is positive. O The reaction will proceed in both directions simultaneously. This reaction can only proceed in one direction and must be the rate limiting reaction for this point in metabolism.arrow_forward23. Calculate the free energy of hydrolysis ATP in a rat liver cell in which the ATP, ADP, and Pi concentrations are 3.4, 1.3, and 4.8 mM, respectively. AG" for this hydrolysis -31.5 kJ/molarrow_forward
- 11. Quantitative Relationships between Rate Constants to Calculate Km, Kinetic Efficiency (kcat/Km) and Vmax - II Triose phosphate isomerase catalyzes the conversion of glyceraldehyde-3-phosphate to dihydroxyacetone phosphate. Glyceraldehyde-3-P dihydroxyacetone-P The Km of this enzyme for its substrate glyceraldehyde-3-phosphate is 1.8 x 10-5 M When [glyceraldehydes-3-phosphate] = 30 μM, the rate of the reaction, v, was 82.5 μmol mL-1 s-1. a. What is Vmax for this enzyme? b. Assuming 3 nm/mL of enzyme was used in this experiment (E total = 3nm/mL) for this enzyme? c. What is the catalytic efficiency (kcat/km) for triose phosphate isomerase? d. Does this enzyme reach catalytic perfection?arrow_forwardThe conversion of dihyroxyacetone phosphate to glyceraldehyde 3-phosphate, catalyzed by triose phosphate isomerase, has an equilibrium constant of Keq’ = 0.0475. Calculate the standard free-energy change for this reaction at 25ºC and pH 7.0.arrow_forwardThe equilibrium constant for the hydrolysis of the peptide alanylglycine (Gly-Ala in the reaction from Part B) by a peptidase is K = 9.0 × 10² at 310 K. Calculate AG for this reaction. Express the Gibbs free energy to three significant figures. AG = Submit ΠΑΠΙ ΑΣΦ Request Answer ? kJ/mol Keq [Gly] [Ala] [Gly-Ala]arrow_forward
- 23. An important step in glycolysis is the formation of ATP and pyruvate from phosphoenol-pyruvate (PEP) and ADP. PEP ADP pyruvate+ATP The equilibrium constant (Keq) for this reaction is approximately 2.5x10°. Calculate standard free energy change (AG°') for this reaction. Show your work. onege b. Is the reaction exergonic or endergonic at standard conditions? If, at equilibrium, the concentrations of ADP and ATP are 0.2 mM and 2.0 mM, respectively, what is the equilibrium concentration ratio of [pyruvate] to [PEP]? Show your work. с.arrow_forwardver is given. A +B C+ D 1) Calculate AG for the above react un and indicáte whether the reaction is favorable or unfavorable [A] = 0.9 M 20°C AG° = 4 KJ/mol %3D [B] = 15mM [C] = 4mM [D] = 3 M R= 8.314 J/moleK %3D %3D 4.7 and explain how you know.arrow_forwardCalculate AG for this reaction under the following conditions: 37°C, pH 7, [Pyruvate] = [CO₂] = 4.0 mM, [OAA] = 2.0 mM, [ATP] = 3.5 mM, [P;] = 5.0 mM, and [ADP] = 1.8 mM. Use 2 signficant figures. AG= i kJ.mol-1 Under these conditions, the reaction is (Tolerance is +/- 2%)arrow_forward
- 6. The AG of the citric acid cycle reaction catalyzed by succinyl-CoA synthetase is approximately 0 kJ/mol. Which of the following statements explains why we would expect this reaction to be highly exergonic, but why the overall AG = 0 in reality. A) The reaction cleaves a thioether linkage, and the released free energy is used to generate a new thioester linkage in a product molecule. B) The reaction cleaves a thioester linkage, and the released free energy is used to generate GTP. C) The reaction cleaves a thioester linkage, and the released free energy is used to generate a new thioester linkage in a product molecule. D) None of the abovearrow_forward7. Representative values of Vm and ApH for the inner mitochondrial membrane and the thylakoid membrane at 25°C are provided in the table. Vm Арн 1.0 pmf Inner mitochondrial 0.166 V 0.2251 membrane Thylakoid membrane O,1483 0.03 V B) What is the value of AG for the thermodynamically feasible (“downhill") movement of 1 mole of H across the inner mitochondrial membrane?arrow_forward3. Respiration is related to how cells balance redox reactions in the metabolic pathway. It can be aerobic if there is plenty of oxygen present or anaerobic if not. In class, we discussed the respiratory quotient for triolein as a carbon source. A. Calculate the respiratory quotient (RQ) for aerobic oxidation of the triglyceride formed by glycerin and three stearic acids (tristearin). Draw the structure and write the stoichiometrically balanced equation for the complete breakdown of the triglyceride by oxidation to CO₂ and water. B. As in part A, draw the structure of sucrose, write the stoichiometrically balanced equation and determine the RQ for it's metabolic breakdown to CO₂ and water. C. Anaerobic respiration occurs when cells operate without dissolved oxygen. List at least 4 different chemicals that act as electron acceptors when oxygen is not present.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON