
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Help
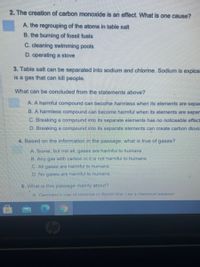
Transcribed Image Text:2. The creation of carbon monoxide is an effect. What is one cause?
A. the regrouping of the atoms in table salt
B. the burning of fossil fuels
C. cleaning swimming pools
D. operating a stove
3. Table salt can be separated into sodium and chlorine. Sodium is explos
is a gas that can kill people.
What can be concluded from the statements above?
A.
ha
ful compound can become harmless when its elements are sepa
B. A harmless compound can become harmful when its elements are separ
C. Breaking a compound into its separate elements has no noticeable effect
D. Breaking a compound into its separate elements can create carbon dioxic
4. Based on the information in the passage, what is true of gases?
A Some, but not all, gases are harmful to humans.
B. Any gas with carbon in it is not harmful to humans.
C. All gases are harmful to humans.
D. No gases are harmful to humans.
5. What is this passage mainly about?
A Germanv's use of chlorine in World War I as a chemical weapon
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Amoxil is an antiblotic used to treat infections of the ear, nošė, and throat. It is sold as a suspension in water. ot to a. What is the weight/volume percent concentration in a suspension of Amoxil that contains 50 mg/mL? b. If the typical pediatric dose is 25 mg/kg per day, what volume of drug is given for a 15-kg child?arrow_forwardL Moving to another question will save this response. Question 18 Which is not an important property of water? a. Solvency b. Adhesion c. Cohesion d. All of the above are important A Moving to another question will save this response. 15,213 FEB 25 Question 18 of 40> 2.5 points Question 18 of 40 tv Save Answer C A ssaarrow_forwardPercocet contains oxymorons at varying strengths. Every strength also contains 325 mg of acetaminophen per tablet. If the dose is not to exceed 3.9 g of actaminophen daily, what is the maximum number of Percocet tablets a patient can take daily?arrow_forward
- Is something wrong here?arrow_forwardipboard Font Paragraph Styles Aspirin Yield Lab NAME: 6. How much aspirin is needed for Part B #1? PRE-LAB 1. What is an antipyretic? REPORT: 2. What is an analgesic? A. Preparation of Aspirin DAY 1 3. What is the "active ingredient" in aspirin? Why is it not ingested directly? 1. Mass of salicylic acid (g) 2. Mass of filter paper (g) 4. In this experiment, 2.00 g of salicylic acid reacts with an excess amount of acetic anhydride. Calculate the theoretical yield of acetylsalicylic acid for this synthesis. 3. Mass of dried filter paper plus sample (g) 4. Theoretical yield of aspirin (g) 5. Experimental (actual) yield of aspirin (g) 5. A0.331 g sample of aspirin prepared in the laboratory was dissolved in 95% ethanol and titrated to a phenolphthalein endpoint with 16.7 ml of 0.107 M NAOH. 7. Volume of water used in the experiment 8. Mass of aspirin dissolved in the experiment (see A7 a. Calculate the moles of acetylsalicylic acid in the aspirin sample. 6. Experimental yield, corrected…arrow_forwardHello can you please help me answer this?arrow_forward
- help mearrow_forwardA doctor has ordered for a week 0.35 grams of Carvedilol for a patient with high blood pressure. If your stock on hand consists of 25 mg tablets, how many tablets will you need for 1 day's treatment for the patient? QUESTION 13 O a. 2 O b. 14 O C. 7 O d. 20 O e. 1 QUESTION 14arrow_forwardThis drug in the picture can treat: O arrhythmia Epilepsy Fever Cancer HO Narrow_forward
- What would step 1 and 2 be?arrow_forwardWhen drugs are absorbed into the body and then eliminated, a graph of concentration in the body vs. time will have the general shape shown at right. Using a drug with a slower elimination rate will _________ the area under the curve. a. decrease b. increase c. not changearrow_forwardQuestion 29 When an analysis finds 2.0 mg of a toxin dissolved in 10. kg of a solution, this equals how many parts per billion (ppb)? A. 2.0 B. 2.0 X 10 to the second power OC. 2.0 X 10 to the 5 power OD. 0.5 X 10 to the 5 powerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY