
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
2
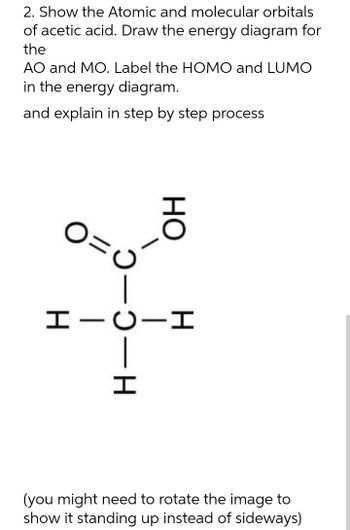
Transcribed Image Text:2. Show the Atomic and molecular orbitals
of acetic acid. Draw the energy diagram for
the
AO and MO. Label the HOMO and LUMO
in the energy diagram.
and explain in step by step process
0=0
HO
HICII
-H
(you might need to rotate the image to
show it standing up instead of sideways)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Just #2 pleasearrow_forwardA 500 mL bottle of water contains 100 ppm of calcium. The recommended daily intake of calcium for teenagers is 1300 mg. How many bottles of water would you need to drink in order get this amount of calcium? (note: the density of water is 1 g/mL)arrow_forwardEvery year Every second (1 year 365 days).arrow_forward
- A runner wants to run 3.9 miles. She knows her running pace is 4.4 km per hour. how many minutes will her run take? Use dimensional analysis.arrow_forwardA A Monate ab 4 Paragraph Help Search (Alt+Q) No Spacing Heading 1 Editing Dictate Sensitivity Voice Sensitwity Styles Two methods of separating an undissolved solid from a liquid are and The method for separating a dissolved solid from the water in an aqueous solution 15 • Using a solvent to dissolve only one substance in a mixture is called 2. A mixture can consist of two or more pure substances. Does that mean a mixture can only consist of elements? 3. Explain your answer to question number 2 4. Oil and water mixed together form a heterogenous or homogenous mixture? 5. 2.000 g of the unknown mixture was placed in an evaporating dish. The empty evaporating dish weighs 38.135 g. After subliming the ammonium chloride from the mixture, the evaporating dish and the remaining mixture weighs 39.775 g. (Put your answer in 3 sig figs and show your work) • What is the weight of the ammonium chloride? • What is the percent of ammonium chloride in the sample? Normal 1. Fill in the following…arrow_forwardAnswer choices for blank 1(there is only one correct answer for each blank): A B C D E F G H J Answer choices for blank 2(there is only one correct answer for each blank): A B C D E F G H J Answer choices for blank 3(there is only one correct answer for each blank): A B C D E F G H J Answer choices for blank 4(there is only one correct answer for each blank): A B C D E F G H J Answer choices for blank 5(there is only one correct answer for each blank): A B C D E F G H Jarrow_forward
- The world population is estimated to be 7.4 x 109. Nauru is the smallest island nation and comprises 1.5 x 10-4% of the world population. If the percentage of left-handed people is approximately 12%, estimate the number of left-handers on the island of Nauru.arrow_forward7. A cube of an unknown metal measures 1.69 mm on one side. The mass of the cube is 31.4 mg. Which of the following is most likely the unknown metal? SHOW your work [5 points] Metal Density (g/ cm- cm3) rhodium 12.4 8.96 copper niobium 8.57 vanadium 6.11 zirconium 6.51arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY