
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:**Reaction Mechanism Explanation:**
**Question:**
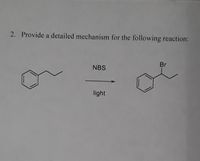
2. Provide a detailed mechanism for the following reaction:
**Reaction Description:**
- **Reactant:** The structure on the left is an alkylbenzene, specifically ethylbenzene, consisting of a benzene ring attached to an ethyl group (C₂H₅).
- **Reagent:** NBS (N-Bromosuccinimide) is used as the brominating agent.
- **Conditions:** The reaction takes place under the influence of light, indicating a radical bromination mechanism.
- **Product:** The structure on the right shows bromination at the benzylic position, resulting in the formation of (1-bromoethyl)benzene.
**Mechanism Overview:**
1. **Initiation:**
- Light provides energy to homolytically cleave the bromine bond in NBS, generating bromine radicals.
2. **Propagation:**
- A bromine radical abstracts a hydrogen atom from the benzylic (methyl) position of ethylbenzene, forming a benzylic radical.
- The benzylic radical reacts with a bromine molecule (Br₂), formed from NBS, to form the brominated product and regenerate a bromine radical.
3. **Termination:**
- Two bromine radicals may combine to form Br₂, or other possible radical combinations may terminate the reaction.
This sequence illustrates the radical chain mechanism typical in NBS-mediated bromination, emphasizing the selectivity for the allylic or benzylic positions due to radical stability.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer.arrow_forward2. Provide a mechanism for the following reaction (show all molecular orbital interactions) ge hv A 2 1arrow_forwardWhat is the complete mechanism using curved arrow formalism of the two products shown below? Explain why one is major and the other is minor product formation.arrow_forward
- Which product(s) will form under the conditions below? Br. -8308 deuterium bromide O A only O B only O Conly O Donly O A and D only OB and C only OA and Conly OB and D only OA, B, C and D Br A D Brarrow_forwardно CH3CO₂Et LDA, -78 °C NaOEt EtOH in addition to the mechanism predict which product is favoredarrow_forward19.) Draw all possible products for the reaction below. Show the mechanism to support your an wer a. b.) Tequivalent HC) I equivater HCIarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY