
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Predict the NMR spectra for each of these two compounds by listing, in the NMR tables below, the chemical shift, the splitting, and the number of hydrogens associated with each predicted peak. Sort the peaks from largest chemical shift to lowest.
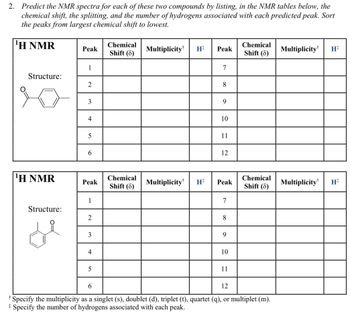
Transcribed Image Text:2. Predict the NMR spectra for each of these two compounds by listing, in the NMR tables below, the
chemical shift, the splitting, and the number of hydrogens associated with each predicted peak. Sort
the peaks from largest chemical shift to lowest.
¹H NMR
Structure:
¹H NMR
Structure:
de
Peak
1
2
دیا
4
5
6
Peak
1
2
3
4
5
Chemical
Shift (8)
Chemical
Shift (8)
Multiplicity H Peak
Multiplicity
H
7
8
9
10
11
12
Peak
7
8
9
10
11
Chemical
Shift (8)
Chemical
Shift (8)
6
12
*Specify the multiplicity as a singlet (s), doublet (d), triplet (t), quartet (q), or multiplet (m).
* Specify the number of hydrogens associated with each peak.
Multiplicity H
Multiplicity H
Expert Solution
arrow_forward
Step 1
We have to complete the given two tables.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. Determine the multiplicity (singlet, doublet, etc) of each signal in the expected 'H NMR spectrum of the following compound. Circle the carbon atom that would be the most deshielded in the 13C NMR spectrum. مله Ν Harrow_forward1. Consider this compound: rCEN CHz CH3 b) How many signals would this compound give in the 'NMR? You may group aromatic hydrogens together as one signal. On the structure above, label the different types of H's as c) А, В, С, etc. hydrogens, their shifts, and the splitting you would observe in the 'NMR spectrum of this compound, for example: In table form, list the peaks, the number of splitting singlet Label # of hydrogens Shift (6) 2.0 etc. ABarrow_forward3) Predict the number, location, integration, and splitting of all peaks that would appear in the proton NMR spectrum of the molecule shown. oarrow_forward
- A.) How many signals does the HNMR of the unknown C6H14O have? B.)Whats the ratio between the number of different hydrogens in the unknown compound C6H14O? C.)Explain splitting patterns of all the signals (in depth if possible the lecture confused me). D.)Explain the chemical shifts of the signals based upon the locations of hydrogens relative to the desheilding groups if any are present in the compound. For A. I think there are 4 signals. For B. I think the ratio is 1:1:3:9 for the hydrogens on the HNMR spectrum. For the last 2 questions (C. and D.) the lecture confused me so an in depth of how you found the answer would be greatly appreciated. Also the unknown for the attached HNMR is C6H14O.arrow_forwardPredict the chemical shift of the following compounds? find out different types of protons and give their approximate chemical shift values based on their shielding and de shielding.arrow_forwardA.) How many signals are seen for the CNMR spectrum of the unknowns C6H14O? B.) Explain the chemical shifts of the signals seen based upon the location of the carbons that are relative to deshielding group if any are seen in the compound (unknown is C6H14O)? For question A. I believe it is 4 signals. For question B. I know that none of the signals are in the deshielding are of the chart but are all located in the shielded region but i am unsure of how to determine the chemical shifts of the given signal. If possible an in-depth explanation would be greatly appreciated in order to understand this better.arrow_forward
- 3. For the structure shown, assign the signals to the respective hydrogen and carbon on the HNMR and CNMR spectra given below. O n Rarrow_forward1) Listen What are multiplicities of the signals for the groups identified, A, B and C in the H NMR Spectrum of the molecule shown below? В CH2 CH3 H3C CH3 C CH3 A Singlet, doublet, triplet Doublet, singlet, quartet Singlet, multiplet, triple Singlet, singlet, singletarrow_forwardArrange the following compounds in their correct order of chemical shift (). 1=Highest and 4=lowest. (All show just one signal in their 1HMR spectrum).arrow_forward
- Predict how many peaks you would expect to observe in the ‘H NMR,spectrum of the compound shown below and indicate the integration value you would expect to see for each peak. Please explain how you got the answer. (It may be useful to label each hydrogen atom using lowercase letters).arrow_forwardHelp pleasearrow_forwardWhich one of the labeled hydrogen atoms in the given molecule will have the greatest chemical shift (furthest downfield)? م شد Ha Ho He Hdarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY