
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
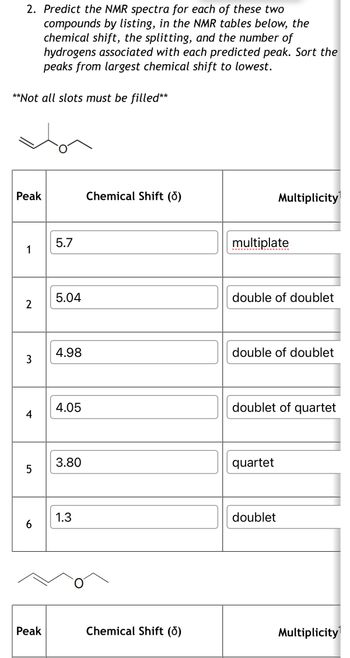
Transcribed Image Text:2. Predict the NMR spectra for each of these two
compounds by listing, in the NMR tables below, the
chemical shift, the splitting, and the number of
hydrogens associated with each predicted peak. Sort the
peaks from largest chemical shift to lowest.
**Not all slots must be filled**
Peak
Chemical Shift (d)
5.7
1
Multiplicity
multiplate
..........
5.04
double of doublet
2
4.98
double of doublet
3
4.05
doublet of quartet
4
5
LO
3.80
quartet
1.3
doublet
6
Peak
Chemical Shift (d)
Multiplicity
Transcribed Image Text:11:14
worksheets.beyondlabz.com
...
Peak
Chemical Shift (d)
Multiplicity
1
2
3
4
LO
5
6
t
Specify the multiplicity as a singlet (s), doublet (d), triplet
(t), quartet (q), or multiplet (m).
キ
Specify the number of hydrogens associated with each peak.
Question 5
5 (a). Using the peak information you listed in the tables for
both structures, assign each peak to that portion of the
structure that produces the peak in the NMR spectrum. Draw
this diagram on your own sheet of paper and attach the sketch
of your drawing to this question
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- A STK2083_Molecular_Spectroscopy_Group Assignment.pdf - Adobe Acrobat Reader DC File Edit View Sign Window Help Home Tools STK2083_Molecular. x Sign In 1 /1 119% a. Which of the compounds below best matches the following 'H NMR spectrum? Integration values are indicated next to their corresponding signal. 2. Search 'Signature' Convert PDF но Edit PDF A B Comment OH HO Combine Files D E EI Organize Pages 24 16 2 Redact A Compress PDF 16 U Protect 8 D Fill & Sign Create, edit and sign PDF forms & agreements PPM b. Briefly explain your answer 2 (a) based on number of signals, position of signals and the integration of the signals. Start Free Trialarrow_forwardPlease don't provide handwriting solutionarrow_forwardCan i get help with this problem pleasearrow_forward
- 10.arrow_forward6. For each compound in the group of isomers, indicate the number of signals you would expect to see in the corresponding ¹H-NMR spectrum, indicate the splitting pattern with s (singlet), d (doublet), t (triplet), quarter (q), pentet (p), sextet (sx) or m (multiplet) each signal, and the integration ratios for the signals. x Number of signals: Splitting pattern: Integration ratio: Number of signals: Splitting pattern: Integration ratio: Number of signals: Splitting pattern: Integration ratio: Can all these isomers be distinguished from one another by these three pieces of information? Explain.arrow_forwardIn a HNMR spectrum, the molecular structure * where A, B and D are different parts that contain no NMR-active atoms, would produce which of the following features? OA Two signals of equal strength, each being a doublet. B. Two signals of equal strength, each being a triplet. OC. Two signals, one three times the strength of the other, with the stronger one being a doublet. D. Two signals, one twice the strength of the other, with the weaker one being a triplet and the other a doublet. OE. One unsplit signal.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,