
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
For each of the following pairs of half-cells:
a. Write a balanced chemical equation for each half-reaction and the overall reaction.
b. Determine E°cell for the reaction.
c. Is the cell spontaneous as written?
d. Calculate ΔG°rxn for the cell reaction.
Anode: Al(s) Al+3(aq) Ered = -0.1238V
Cathode: I2(aq) I-1(aq) Ered = +0.5273V
Anode: NO3-1(aq) HNO2(aq) Ered = +0.9513V
Cathode: Cr+3(aq) Cr(s) Ered = +0.7237V
Anode: ClO4-1(aq) Cl2(aq) Ered = +1.2407V
Cathode: Cr2O7-2(aq) Cr+3(aq) Ered = +1.2315V
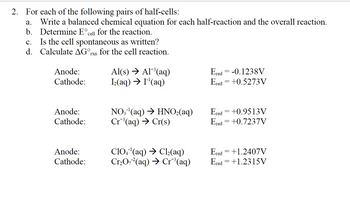
Transcribed Image Text:2. For each of the following pairs of half-cells:
a. Write a balanced chemical equation for each half-reaction and the overall reaction.
b. Determine Eºcell for the reaction.
C. Is the cell spontaneous as written?
d. Calculate AGO xn for the cell reaction.
Anode:
Cathode:
Anode:
Cathode:
Anode:
Cathode:
Al(s) → Al*³(aq)
L₂(aq) → I¹(aq)
NO:¹¹(aq) → HNO₂(aq)
Cr¹³(aq) → Cr(s)
ClO4¹(aq) → Cl₂(aq)
Cr₂O²(aq) → Cr*³(aq)
Ered
-0.1238V
Ered=+0.5273V
Ered=+0.9513V
Ered=+0.7237V
Ered +1.2407V
Ered +1.2315V
=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A battery can provide a current of 3.40 A at 2.80 V for 8.50 hr. How much energy (in kJ) is produced? Energy = kJarrow_forwardConsider the galvanic cell based on the following half-reactions: Zn+ + 2e + Zn e° = -0.76 V Cd²+ + 2e → Cd e° = -0.40 V a. Determine the overall cell reaction and calculate e (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (5). If a box is not needed, leave it blank.) b. Calculate AG° and K for the cell reaction at 25°C. AG kJ K = [Zn**] = 0.10 M and [Ca] = c. Calculate e cell at 25°C when Zn 1.2 x 10-5 M. Ecell =arrow_forwardO ELECTROCHEMISTRY Designing a galvanic cell from tw... A chemist designs a galvanic cell that uses these two half-reactions: standard reduction half-reaction 2H₂O(1)+2e Write a balanced equation for the half-reaction that happens at the anode. Ered= Br₂(1)+2e Ered = +1.065 V Answer the following questions about this cell. Write a balanced equation for the half-reaction that happens at the cathode. Write a balanced equation for the overall reaction. that powers the cell. Be sure the reaction is spontaneous as written. Do you have enough information to calculate the cell voltage under standard conditions? If you said it was possible to calculate the cell voltage, do so and enter your answer here. Round your answer to 3 significant digits. 0 0 0 O Yes O No H₂(g) + 2OH(aq) 2 Br (aq) V potential = -0.83 V - 3 ⠀ ローロ X 2 5 x10 Sarrow_forward
- AG° = - â F E° ćel! n FE Consider the following reaction: 3 Pb2+ + 2 Cr(s) → 3 Pb(s) + 2 Cr3+ Using the values in Table 17.2, calculate Eell for this reaction. How many electrons are transferred in this reaction? n = Calculate AG° for this reaction. Is the reaction spontaneous under standard conditions? Justify your answer. Cell Potential and Keg We can also relate Ecell to Keq by the following equation: Ecell = RT nF %3D Consider the oxidation of zinc metal by acid (H*) under standard conditions (25°C). Write a balanced equation for this reaction. Using the values in Table 17.2, calculate Eell for this reaction. How many electrons are transferred in this reaction? n = Calculate Keg for this reaction.arrow_forward3. Calculate the free energy, AG°, for each electrochemical cell given the following information and determine if the reaction is spontaneous or nonspontaneous. T= 298 K a. E cell =+ 2.10 V, n= 2 b. Каg— 2.2 х 10-3 c. E cell =- 0.677 V, n = 3 =arrow_forwardCell Potential For a given galvanic cell, the standard cell potential can be calculated by subtracting the standard half-cell potential of the reaction that occurs at the cathode from the standard half-cell potential of the reaction that occurs at the anode: Ecell Ered (cathode) - Ed (anode) ▼ Tarnish on copper is the compound CuO. A tarnished copper plate i placed in an aluminum pan of boiling water. When enough salt is added so that the solution conducts electricity, the tarnish disappears. Imagine that the two halves of this redox reaction were separated and connected with a wire and a salt bridge. Part A Calculate the standard cell potential given the following standard reduction potentials: A²+ +3e Cu²+ + 20 Express your answer to two decimal places and include the appropriate units. ▸ View Available Hint(s) Ecell = li Value 4 → Units ? Al; E Cu; E 19 of 42 -1.66 V 0.340 V Review | Constants | Periodic Tablearrow_forward
- 11. The following two half-cells are connected to form a full Galvanic cell: Cu"(aq) +2e Au (aq) +e → Cu(s) → Au(s) E° = 0.34 V E 1.69 V a. Calculate E° for the cell. b. Give the cell notation for this system. c. Give the balanced cell reaction. d. Calculate AG for the reaction e. Calculate Keg for the reaction. £. Calculate the cell potential if the activity of Cu is 0.0100 and the activity of Au" is 1.340.arrow_forwardAs with any voltaic cell, the potential of zinc-copper cell changes during cell operation because the concentrations of the components do.If E°cell = + 2.71 V, [Mg2+] = 1.0 x 10-4 M and [Cu2+] = 2.0 M; find Ecell.Is the reaction at equilibrium?arrow_forwardA charger that supplies a current of 120. mA of current is used to recharge a NiCd battery. How much time, in h, does it take to convert 1.55g of Cd(OH)2 back into Cd so the battery can be used again? O4.73 O 1.25 O 2.29 3.94 O0.732arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY