
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please do all parts of the question
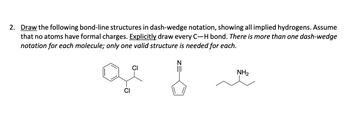
Transcribed Image Text:2. Draw the following bond-line structures in dash-wedge notation, showing all implied hydrogens. Assume
that no atoms have formal charges. Explicitly draw every C-H bond. There is more than one dash-wedge
notation for each molecule; only one valid structure is needed for each.
ge
|||
NH₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Can you do the dash-wedge bonds with the C-H bonds explicitly drawn as well, please
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Can you do the dash-wedge bonds with the C-H bonds explicitly drawn as well, please
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is wrong with the following statement? "The results of the experriment do not agree with the theory. something must be wrong with the experiement"arrow_forwardNeed help with this question. Thank you :)arrow_forwardThe distillation flask is best completely filled with mixture as large as available never less than half empty about 70% fullarrow_forward
- Express the following points given in Cartesian coordinates in terms of spherical coordinates. (x, y, z): (1, 0, 0); (0, 1, 0); (0,0,1); (0,0,-1)arrow_forwardI don't understand How to do this question and I am on my last try please help me!arrow_forward1-Pentanol to 1-bromopentane Chemicals: - 60ml Conc. Sulfuric Acid - 100ml Saturated Sodium bicarbonate - 65ml 1-Pentanol - 78g sodium bromide - Distilled water - 58.42g 1-Bromopentane 1-Pentanol Sodium Bromide Sulfuric Acid 1-Bromopentane Formula C5H12O NaBr H2SO4 C5H11Br MW (g/mol) 88.15 102.894 98.078 151.04 Density (g/mL) 0.811 3.21 1.84 1.218 Boiling point (*C) 138 1,396 337 130 NaBr(aq) + H2SO4(aq) -> NaHSO4(aq) + HBr(aq) CH3(CH2)4OH(aq) + H+ Br- (aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4Br(aq) + H2O(aq) How do I calculate the percent yield and identify the limiting reagent?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY