
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
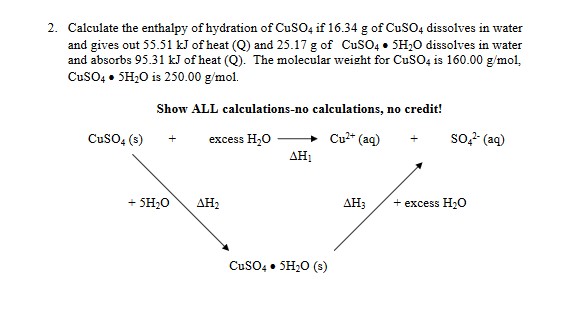
Transcribed Image Text:2. Calculate the enthalpy of hydration of CuSO, if 16.34 g of CUSO, dissolves in water
and gives out 55.51 kJ of heat (Q) and 25.17 g of CuSO4 • SH;O dissolves in water
and absorbs 95.31 kJ of heat (Q). The molecular weight for CusO, is 160.00 g/mol,
CusO, • SH;0 is 250.00 g/mol.
Show ALL calculations-no calculations, no credit!
CusO, (s)
excess H,O
Cu" (aq)
so- (aq)
AH1
+ SH;0
АНа
АНа
excess H;0
CusO4 • SH20 (s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8:35 A moodle.nct.edu.om P Flag question Answer the following questions by using mole concept. (a) Calculate the molarity of a 105 g NazSO4 in 570 ml solution. [Atomic mass: Na=23 g/mol, S= 32 g/mol, O=16 g/mol, C=12 g/mol, H= 1 g/mol] (a1)no of moles of Na,SO4 (a2) molarity of solution (b) 29 g C6H12Og is dissolved in 210 g of water. Find out the molality of the solution. (b1) no of moles of CeH1206 (b2) molality of solution (c) How much volume of 1.9 M NaOH solution can be made by diluting 74 ml of 4 M NaOH. (d) Aspirin (CgHgO4) is a medication used to reduce pain, fever, or inflammation. Find out the mass of 3.5x1023 molecules of aspirin in gram. (d1) no of moles of Aspirin (d2) mass of aspirin Question 28 -> 11 •.. +arrow_forwardIf the solution mixture becomes colder, which of the following statements is true? The reaction is endothermic and AH > o The reaction is exothermic and AH > o The reaction is endothermic and AH < 0 The reaction is thermoneutral and AH = 0 The reaction is exothermic and AH < 0arrow_forwardA student weighs out 0.213 g MgCl2·H2O (Mw = 131.235 g mol-1) of and 0.167 g of NaOH (Mw = 39.997 g mol-1 ) and dissolves both these in DI water to make up two separate solution. What are the mole amounts of both these reagents and which is the limiting reagent?arrow_forward
- When mixing sodium chlorate solution (volume 1.0L, density 1.1614 g/ml, mass fraction 22%) with hydrochloric acid( 2L, density 1.0980 g/ml, mass fraction 20%) an antiseptic substance was released. Write and balance the Mentioned reaction and name this anticipatic substance.arrow_forwardA solution was prepared by dissolving 0.687 g of sulfur, S, in 100.0 g of acetic acid, HC2H3O2. Calculate the freezing point and boiling point of the solution. (K, = 3.08°C/m and Kf = 3.59°C/m, T, = 118.5°C and T† = 16.60°C) Freezing point: °C Boiling point: °Carrow_forwardUse the following atomic masses (in g/mol): Mg = 24.31; O = 16; Ca = 40.08; C = 12.01; Na = 23; H = 1; N = 14.01; S = 32.06; Cl = 35.45 In the assay of NaHCO3, 3.0g of the solid is dissolved in 25mL water. What is the normality? How many mL of 1N H2SO4 will be required to neutralize this solution? From this volume of acid, compute the percent purity of NaHCO3.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY