
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Here is the solution to the problem. However, I do NOT understand the solution. Please explain the answer in a clear step-by-step fashion to me. Explain the answer provided in the image in a clear fashion and as if I did not know much about organic chemistry.
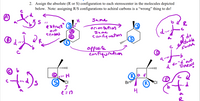
Transcribed Image Text:2. Assign the absolute (R or S) configuration to each stereocenter in the molecules depicted
below. Note: assigning R/S configurations to achiral carbons is a “wrong" thing to do!
Same
e xtruct S
out
senters
orien tation
a
sume
confiquation
I do
Opposite
confiquration
double
Switch
© b
|||||
E"d"out
d
DARN!
CI
R
Bril
R CI
2 = 17
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help in answering the organic chemistry questions given below. Explanations are welcome.arrow_forwardOrganic Chemistry 1 Here is the professor's solution to the problem. The assignment has been turned in and the answer key has been shared with the class. So, this is the official answer key. However, I do NOT understand the solution. Please explain the answer in a clear step-by-step fashion to me. Explain the answer provided in the image in a clear fashion and as if I did not know much about organic chemistry. Question: Identify the isomeric relationship between the molecules of each pair depicted below. Your choices are dieastereomers, enantiomers, identical, or constitutionalarrow_forwardFor each of the oxygen-containing functional groups mentioned in the lecture slides in this module, name or describe or draw or give the formula of the smallest molecule that contains that functional group. (Hopefully you will give the name AND some kind of representation of those molecules. Feel free to post a pic that you take of your drawing from your phone.) Hint: Many of these molecules might appear in the "Jeopardy questions on Organic and Carbohydrates" slides that are posted ahead in the Carbohydrates module.arrow_forward
- Organic Chemistry Маxwell Draw the organic and inorganic products for the following acid/base reaction. H .N. Н. 2 products H.arrow_forwardData for Problem 2 ZOO 180 150 120 140 100 80 770 дом -8 60 40 20 10 9 a 5 015 100 80 Relative Intensity 8 ส 20 20 40 50 ppm septet 3 2 60 70 80 90 100 m/zarrow_forwardOrganic Chemistry 1 Here is the professor's solution to the problem. The assignment has been turned in and the answer key has been shared with the class. So, this is the official answer key. However, I do NOT understand the solution. Please explain the answer in a clear step-by-step fashion to me. Explain the answer provided in the image in a clear fashion and as if I did not know much about organic chemistry. Question: Identify the isomeric relationship between the molecules of each pair depicted below. Your choices are diastereomers, enantiomers, identical, or constitutional. Explain your answer without using Fischer projections.arrow_forward
- Organic Chem. This CANNOT be hand-drawn, explanation and illustrations must be typed or digital. if it is hand-drawn your answer will be incomplete and marked as wrong. Molecule: Benzocaine List and explain common industrial or medical applications. what ways can you synthesize this? identifyand discuss the differences. List out any specificprocedural details. Describe the theories behind any method used in asynthesis to create this molecule. (Reactions, techniques,instrumentation, etc.).■For example, if distillation could be used to purify your product, why.arrow_forward10. Your plane crashes in the Rocky Mountains. Miraculously you survive, and find yourself stranded on a deserted mountain slope. While out gathering berries, you stumble across an abandoned but well-stocked organic laboratory! Mentally thanking your chemistry teacher, you immediately begin making the useful compounds listed below. (Show equations with structures and names and give type of reaction) a) octyl methanoate (a hut deodorizer which smells like oranges) Type of Reaction: Structures and Names:arrow_forward4. Below are the structures of some medications. Circle and identify functional groups in the structures. H₂C H₂C core MDPV Mephedrone 'N H CH3 Cocaine CH3 CH3 Methylone CH3 Aom CH3 CH3 CH3 Amphetamine NH₂ но... HO HO LaH O ACO OH 10-deacetylbaccatin IIIarrow_forward
- From what we know today, what do the two Kekulé structures for benzene really represent? August Kekulé was the first person to propose a viable structure for benzene in 1865. Structures that can be separated at low enough temperatures (near absolute zero). Structures that are in a state of rapid equilibrium. O Structures that are in resonance. Structures that are conjugated trienes (--C=C--C%3DC--C3C--).arrow_forwardОН MCPBA NaHCO3, H₂O 1) Dicyclohexylborane 2) NaOH, H2O2arrow_forwardhelparrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY