
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
please explain all the steps
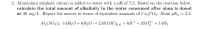
Transcribed Image Text:2. Aluminium sulphate (alum) is added to water with a pH of 7.2. Based on the reaction below,
calculate the total amount of alkalinity in the water consumed after alum is dosed
at 30 mg/L. Report the answer in terms of equivalent amounts of Ca2CO3. Alum pKa = 3.3.
а
Al2(SO4)3 · 14H2O+6H2O → 2A1(OH)3(s) + 6H+ + 3SO;- + 14H2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pressure Vs. Volume 4.500 4.000 3.500 3.000 2.500 2.000 1.500 1.000 0.500 0.000 0.00 5.00 10.00 15.00 20.00 25.00 Volume (cm3) Using the data given the graph above what values would you plot to make a straight line or "linearize" the data from the graph. Choose ONE O V vs. P O 1/V vs. 1/P O V vs. 1/P Pressure (atm)arrow_forwardHelp me to use the convert factorarrow_forwardHow much faster would He gas effuse than Ar? (record your answer to 2 decimal places)arrow_forward
- 2.-I have 48 liters of in a piston at a temperature of 215 °C. If I cool the gas until the volume gas decreases to 34 liters, what will temperature of the gas be in °Carrow_forwardisopropyl alcohol evaporated from a beaker at the rate of 1.057ml/min. How many seconds would it take for 3.70ml of isopropyl alcohol be lost?arrow_forwardA balloon filled with helium has a volume of 38.8 L at 275 K. What volume will the balloon occupy at 243 K?V=?arrow_forward
- . How would the graph change if it were for another substance (other than water)?arrow_forwardA tank with 43.2 kg of water is leaking at a rate of 0.0135 kg/s. How many hours will it take until the tank is completely empty?arrow_forwardUse the kinetic theory to explain the reason materials are more dense in the solid state than in the liquid state. WWhich common liquid is an exception to this rule? Explain what must happen to cause this exception. Use the paperclip button below to attach files. * Student can enter max 3500 characters В I U %3D 65°F Cloudy ^ Type here to searcharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY