Question
do question 2 PLS thank u,
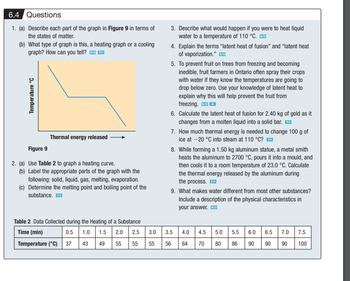
Transcribed Image Text:6.4 Questions
1. (a) Describe each part of the graph in Figure 9 in terms of
the states of matter.
(b) What type of graph is this, a heating graph or a cooling
graph? How can you tell? K/U™I
Temperature °C
Thermal energy released
Figure 9
2. (a) Use Table 2 to graph a heating curve.
(b) Label the appropriate parts of the graph with the
following: solid, liquid, gas, melting, evaporation.
(c) Determine the melting point and boiling point of the
substance. ™
Table 2 Data Collected during the Heating of a Substance
Time (min)
1.0 1.5 2.0
43 49 55
0.5
Temperature (°C) 37
2.5
3.0
55 55
3. Describe what would happen if you were to heat liquid
water to a temperature of 110 °C. /
4. Explain the terms "latent heat of fusion" and "latent heat
of vaporization." IK/U
5. To prevent fruit on trees from freezing and becoming
inedible, fruit farmers in Ontario often spray their crops
with water if they know the temperatures are going to
drop below zero. Use your knowledge of latent heat to
explain why this will help prevent the fruit from
freezing. Ku
6. Calculate the latent heat of fusion for 2.40 kg of gold as it
changes from a molten liquid into a solid bar. ™
7. How much thermal energy is needed to change 100 g of
ice at -20 °C into steam at 110 °C? ™
8. While forming a 1.50 kg aluminum statue, a metal smith
heats the aluminum to 2700 °C, pours it into a mould, and
then cools it to a room temperature of 23.0 °C. Calculate
the thermal energy released by the aluminum during
the process. ™
9. What makes water different from most other substances?
Include a description of the physical characteristics in
your answer. IK/U
3.5
56
4.0 4.5 5.0 5.5 6.0
64 70
80 86 90
6.5 7.0 7.5
90 90 100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
arrow_back_ios
arrow_forward_ios