
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
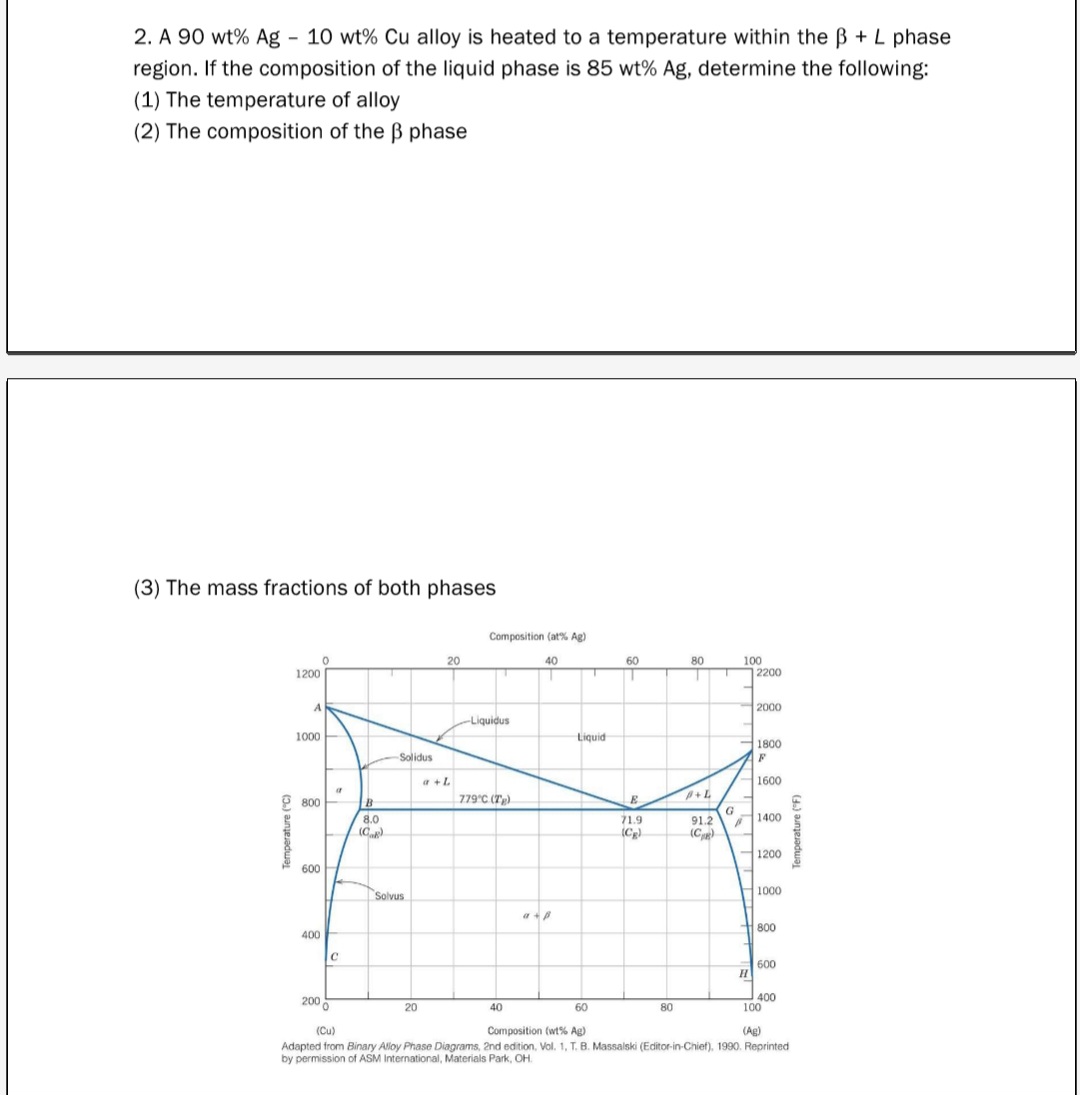
Transcribed Image Text:2. A 90 wt% Ag - 10 wt% Cu alloy is heated to a temperature within the ß + L phase
region. If the composition of the liquid phase is 85 wt% Ag, determine the following:
(1) The temperature of alloy
(2) The composition of the ß phase
(3) The mass fractions of both phases
Temperature (°C)
1200
A
1000
800
600
0
400
a
C
с
2000
B
8.0
(C)
T
Solidus
Solvus
20
20
a + L
Composition (at % Ag)
40
T
Liquidus
779°C (7g)
ap
40
T
Liquid
60
T
E
71.9
(C₂)
80
T
80
P+L
91.2
(C)
G
100
UN
#
H
2200
2000
1800
F
1600
1400
1200
1000
800
60
Composition (wt% Ag)
(Cu)
(Ag)
Adapted from Binary Alloy Phase Diagrams, 2nd edition, Vol. 1, T. B. Massalski (Editor-in-Chief), 1990. Reprinted
by permission of ASM International, Materials Park, OH.
600
400
100
Temperature (°F)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 6 images

Knowledge Booster
Similar questions
- A one-inch thick steak initially at room temperature is placed inside an oven maintained at 250 o C. Estimate how long it will take for its internal temperature to reach 70 o C. What will be the surface temperature at that time if we assume no steam generation? Include radiation as well as convection (h ~ 3 W/m²/K) in your analysis.arrow_forwardThere's a copper calorimeter container with the mass 0.2 kg and has 0.32 kg of H2O and also 0.036 kg of ice (thermal equilibrium at atm). If we drop 1.5 kg of lead at a temperature of 200 Celsius into calorimeter container, find the final temperature (in Celsius). Lead=131J/kgK Copper=390J/kg.K H2O = Cw = 4190 J/kg.K & LF = 3.34 x 105 J/kgarrow_forward4-2arrow_forward
- 1. Consider a blast furnace which is charged with iron ore, coke and flux with the following composition: Iron ore (weight %): Fe2O3-78, SiO2-8.4, MnO=0.6, Al2O3-5.0, P₂Os=1.7, MgO=1.2 and H2O=5.1 Coke (weight %): C=88, SiO2-9, Al2O3-1 and H₂O=2 Flux (weight %): CaCO3-96%, MgCO3-2% and SiO2=2% And pig iron is produced with the following composition: Pig iron (weight %): Fe =92.7, C=4, Si=2, P=0.9 and Mn=0.4 The coke and flux rates are 900 and 425 kg/ton of pig iron, respectively. During smelting 99.5% of Fe is reduced and 0.5% is slagged. The CO/CO2 ratio in the top gas is 2/1. Calculate a) Weight of iron ore b) Weight and composition of slag c) Volume of air required d) Volume and %composition of exit gas.arrow_forwardResearch for the boiling point of the following mixture of compounds. Encircle which would boil first. Finally, indicate what type of distillation will you use to separate them.arrow_forwardGiven the solidus and liquidus temperatures for a copper-gold. Construct the phase diagram for the system and label each region.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The