
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:2. A 0.5 m³ tank holds a two-phase liquid-vapor mixture of carbon dioxide (CO₂) at -17 °C. The quality of the
mixture is 0.6. For saturated carbon dioxide at −17°C, vƒ= 0.983 × 10¯³ m³/kg and vg = 1.756 × 10-² m³/kg.
Determine: (a) the specific volume of the two-phase CO2 as a closed system; (b) determine the total mass of the
CO2; (c) determine the mass of saturated liquid and the saturated vapor, respectively; (d) determine the physical
volume of the vapor and the physical volume of the liquid, respectively; (e) determine the ratio of the physical
volume of the vapor to that of the liquid. This ratio should reflect the fact that the liquid occupies only a very
small fraction of the total physical volume of the tank. Show full steps; carry all units.
Expert Solution
arrow_forward
Step 1

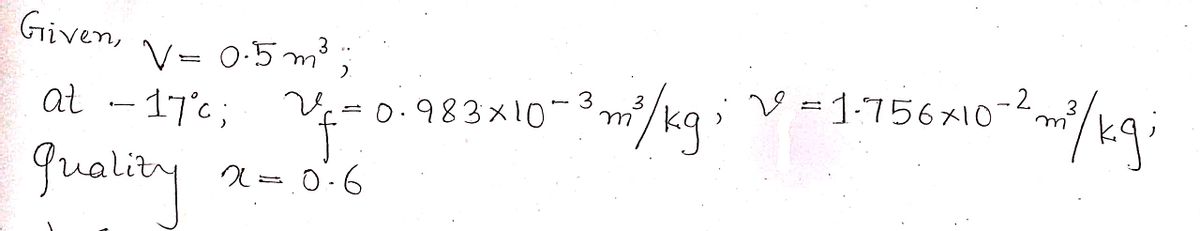
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Q8/A two-phase liquid-vapor mixture of a substance has a pressure of 150 bar and occupies a volume of 0.2 m³. The masses of saturated liquid and vapor present are 3.8 kg and 4.2 kg, respectively. Determine the mixture specific volume in m/kg.arrow_forwardDetermine the quality, in percent, of a liquid-vapor mixture at 3 bars and a specific volume of 0.4 m³/kg. 66.0 а. с. 77.0 b. 71.0 d. 83.0arrow_forwardH.W # (3) Q1: An 80-L vessel contains 4 kg of refrigerant-134a at a pressure of 160 kPa. Determine (a) the temperature, (b) the quality, (c) the enthalpy of the refrigerant, and (d) the volume occupied by the vapor phase. Ans.: 215.6 °C, 0.157, 64.1 kJ/kg, and 0.0776 m Q2: 0.1 kg of water is contained within a piston-cylinder assembly at 100°C. The piston is free to move smoothly in the cylinder. The local and nogalarotion of grouitu ore 100 kPa and 0 S1 m/c? 2981 m atmosnarrow_forward
- A rigid tank contains saturated liquid - vapor water at 85oC. For this state, the specific volume, in m3/k, is measured as 1.9808. The mixture's quality is most likely: 50% 40% 60% 0% 70% 100%arrow_forwardRefrigerant-134a Saturated vapor Saturated liquid A tank occupied by saturated liquid-vapor mixture of refrigerant-134a at 1.8 MPa. If the fraction of the total volume occupied by vapor (Vsaturate vapor/Vtotal) is 0.96, determine quality of the mixture. Present your result cent. (For example, if you find 0.952, enter your result to system as 95.2)arrow_forwardWater is contained in a closed, rigid, 0.25 m3 tank at an initial pressure of 500 kPa and a quality of 80%. Heat transfer occurs until the tank contains only saturated vapor. Determine: (a) the fifinal pressure, in bar. (b) amount of heat transferred to the system.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY