
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
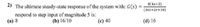
Transcribed Image Text:2) The ultimate steady-state response of the system with: G(s)
8(4s+2)
%3D
respond to step input of magnitude 5 is:
(a) 8
(382+2s+10)
(b) 16/10
(c) 40
(d) 16
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Q2: A mercury thermometer having a time constant of 0.1 min is placed in a temperature bath at 100°F and allowed to come to equilibrium with the bath. At time t=0, the temperature of the bath begins to vary sinusoidally about its average temperature of 100°F with an amplitude of 2°F If the frequency of oscillation is 10/π cycles/min, What is the phase lag? In terms of the symbols used T = 0.1 1≥0 f= 10 元 ==100 x(t) 100+ 2 sin(wt)) السائل SLOWarrow_forwardHelp with the following questionarrow_forwardQ.1 Find the solution of following ODE by Laplace transform of the function x( t ) and Ca(t) that satisfies the differential equation and initial conditions. (b) 3 d²x dt² dx - x = 2 dt -2.5 d at x(0) = 1arrow_forward
- MULTIPLE CHOICE -The answer is one of the options below please solve carefully and circle the correct option Please write clear .arrow_forwardSoces BME 101 Introduction to Biomedical Engineering Shown here is the Hawking equation that describes radiation from black holes nAkc 2hG Where: S-entropy (J/K) A- area of the event horizon (m2) k- Boltzman's constant (m kg/s' K) C- speed of light h-Planck's constant (m? kg/s) a) Using the equation and units of the other terms, find the units for c. b) In this system of units, c = 2.99 x 10°. Convert this to miles/hour. G- universal gravitation constant (m'/kg s') 50°C is d 50°C is d 50°C is d S= 4.18 s= 4.18 5= 4.18 •..arrow_forwardIn the equilibrium-stage model equations, are K-values and enthalpies counted as variables? Are the equations used to compute these properties counted as equations?arrow_forward
- 7.1. A step change of magnitude 4 is introduced into a system having the transfer function Y(s) 10 X(s) s² + 1.65 +4 Determine (a) Percent overshoot (b) Rise time (c) Maximum value of Y(t) (d) Ultimate value of Y(t) (e) Period of oscillationarrow_forwardf & g domain & range pleasearrow_forwardOnly typed solution.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The