
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
show the mechanism of the following reaction step by step. Via Fischer esterification and transesterification.
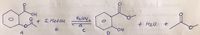
Transcribed Image Text:H2504.
+ 2 MetOH
+ HzO +
OH
A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Formic acid, HCOOH, is found in ants. Write a balanced chemical equation to represent why an aqueous solution of formic acid is acidic.arrow_forwardFind the value of Kb for the conjugate base of the following organic acids. (a) picric acid used in the manufacture of explosives; Ka = 0.16 (b) trichloroacetic acid used in the treatment of warts; Ka = 0.20arrow_forwardHO -OH Oxalic Acid, H₂C₂O₁ PK₁₁ = 1.27 pka2 = 4.266 H₂Ox→ HOX → Ox ²- H₂A → HA → A²- pH = ? Adding 0.20 mole of NaOH to 0.40 mole of NaHC₂O4 (s) with total volume of 500 mL. pH = ? 150 g of NaHC₂O4 (s) dissolved in DI water total volume of 500 mL. pH = ? Adding 0.20 mole of HCI to 0.40 mole of NaHC₂O4 (s) with total volume of 500 mL. pH = ? 0.40 Adding 0.20 mole of HCI mole of Na₂C₂O4 (s) with total volume of 500 mL. pH = ? Adding 0.40 mole of HCI to 0.40 mole of Na₂C₂O4 (s) with total volume of 500 mL. pH = ? Important Key Concept Exercisearrow_forward
- Fill in the left side of this equilibrium constant equation for the reaction of diethylmethylamine (C3H13N), a weak base, with water. D= K, oloarrow_forwardComplete the balanced chemical reaction for the following weak base with a strong acid. In this case, write the resulting acid and base as its own species in the reaction.arrow_forwardidentify the conjugate acid base pairs in this reactionarrow_forward
- What is the reaction of the weak base C5H5N with water? O C5H5N + H2O → C5H5NH" + OH" O C5H5N + H20 → C5H5NH* + H3O* O C5H5N + H20 → C5H5NH* + OH¯ C5H5N + H20 → C5H4N° + H30+arrow_forwardThe acid dissociation constant K of trimethylacetic acid (HC(CH³)₂CO₂) is 9.33 × 10¯6. a Calculate the pH of a 3.6M solution of trimethylacetic acid. Round your answer to 1 decimal place. pH = - 0 X Śarrow_forwardUse Table 18.b or Apendix C to calculate the pH of 0.42 M aniline?arrow_forward
- Phenols are aromatic rings with an alcohol functional group attached directly to the ring. These compounds have unique acidity and solubility for alcohol groups. Phenolic functional groups are weak acids. Predict the solubility of the conjugate base of phenol in water.arrow_forwardConsider the following data on some weak acids and weak bases: acid base Ba K, name formula name formula hydrocyanic acid HCN 10 4.9 x 10 methylamine| CH3NH2 |4.4 × 10 HCH,CO2 1.8 × 10 ethylamine C2H5NH2|6.4 × 10 acetic acid Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution pH 0.1 M NaCN choose one 0.1 M KBr choose one 0.1 М КСН3СО2 choose one 0.1 М CН3NHзBr choose onearrow_forwardIdentify the reactant acid and base in each of the following reactions by letters. 1. CHỊCH,O-CH, CHy + a The acid is The base is 2. H The acid is The base is H H + Na H b BF₂ b BF3 CHỊCH, TO–CHỊCH, H 0= с Na H₂arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
