
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
H2O equations
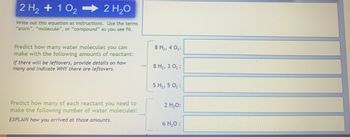
Transcribed Image Text:2 H₂ + 102
2 H₂O
Write out this equation as instructions. Use the terms
"atom", "molecule", or "compound" as you see fit.
Predict how many water molecules you can
make with the following amounts of reactant:
If there will be leftovers, provide details on how
many and indicate WHY there are leftovers.
Predict how many of each reactant you need to
make the following number of water molecules:
EXPLAIN how you arrived at those amounts.
8 H₂, 4 0₂:
8 H₂, 3 0₂:
5 H₂, 5 0₂:
2 H₂O:
6 H₂O:
Expert Solution
arrow_forward
Step 1
The balanced chemical equation of forming H2O is
2H2 + 1 O2 -------> 2H2O
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many grams of H2O are produced if 43.2 g of O2 are consumed in the following reaction? C3H8 + 5 O2 → 4 H2O + 3 CO2 a.)19.5 g b.)24.3 g c.)43.2 g d.)216. garrow_forwardWrite.a balanced equation when hydrogen gas reacts with oxygen gas to produce gaseous water. HTML Editorarrow_forwardIn 2014, the World Anti-Doping Agency announced that Xenon gas would be added to the list of banned substances for athletes. Apparently since 2004, Russian athletes had been inhaling xenon while training, with the physiological effect being to boost the capacity of blood to carry oxygen needs for aerobically demanding sports. If an athlete inhaled an average of 1.75 x 10-4 moles of Xe per breath while sleeping, with an average rate of 13.0 breaths per minute for 8.50 hours how many total GRAMS of Xe(g) were inhaled while sleeping? please show all calculationsarrow_forward
- How to classify each equation as eitherarrow_forwardWhich of the following is a gas-evolution reaction? A. 2 H2(g) + O2(g) → 2 H2O(g) B. None of the above are gas-evolution reactions. C. 2 C2H6(l) + 7 O2(g) → 4 CO2(g) + 6 H2O(g) D. NH4Cl(aq) + KOH(aq) → KCl(aq) + NH3(g) + H2O(l) E. MgSO4(aq) + Ba(NO3)2(aq) → Mg(NO3)2(aq) + BaSO4(s)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY