
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:2) Flash Distillation (or Single-Stage Contact)
A mixture of 60 mole % n-heptane and 40 mole % toluene is vaporized at 101.325 kPa (absolute) until 40
moles of vapor and 60 moles of liquid in equilibrium with each other are produced. This occurs in a single-
stage system and the vapor and liquid are kept in contact until equilibrium is achieved. Calculate the
composition of the vapor and liquid.
Note: Assume the system exhibits ideal behavior (which it mostly does), e.g. Raoult's Law applies and an
Antoine's Vapor Pressure correlation is a sufficiently accurate model of component vapor pressure.
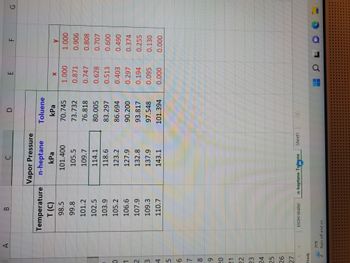
Transcribed Image Text:0
1
2
3
4
5
LO
6
7
8
9
20
21
22
23
24
25
26
27
Ready
A
B
Temperature
T (C)
98.5
99.8
101.2
102.5
103.9
105.2
106.6
107.9
109.3
110.7
EtOH-Water
71 F
Rain off and on
C
Vapor Pressure
n-heptane
kPa
101.400
105.5
109.7
114.1
118.6
123.2
127.9
132.8
137.9
143.1
n-heptane-Toluene Sheet1
D
Toluene
kPa
70.745
73.732
76.818
80.005
83.297
86.694
90.200
93.817
97.548
101.394
E
X
1.000
0.871
0.747
0.628
0.513
0.403
0.297
0.194
0.095
0.000
F
Y
1.000
0.906
0.808
0.707
0.600
0.490
0.374
0.255
0.130
0.000
HO COLO
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 4 images

Knowledge Booster
Similar questions
- A single effect evaporator is used to concentrate 10 000 kg / hr of tomato juice from 5% total solids to 30% total solids. The juice enters the Evaporator at 20 ° C. The evaporator is operated on Steam (80% quality) at 143.27 kPa. The vacuum in the evaporator allows the juice to boil at 75 ° C. The specific heat of the dilute material is 4.1 kJ / (kg ° C) and the concentrate product is 3.1 kJ / (kg ° C). Count it a. Steam demand rate = ..... kg / hour. b. Steam economy when condensate temperature is released at 75 ° C. = .... (kg of water evaporated / kg of steam)arrow_forwardShow calculation alsoarrow_forwardFruit juices at 25 °C containing 5% total solids were concentrated via a single effect evaporator. The evaporator is operated in a vacuum at an evaporation temperature of 80 °C, and 85% quality steam is supplied at 169.06 kPa. The desired concentration of the final product is 40% total solids. The rate of concentrated product leaving the evaporator is 3000 kg / hour. The specific heat of fruit juice is 4.05 kJ / (kg °C), and the concentrated product 3.175 kJ / (kg °C). Count : a. The required steam rate is = Answer kg / hour. b. Steam economy if the condensate temperature cannot be released at 90 °C. = Answer (kg evaporated air / kg vapor)arrow_forward
- A feed column has the composition given in the table below, and it at as a pressure of 30 bar and at a temperature of 400C. Calculate the flow of the liquid and vapor phases. Feed kmol/h ethane 20 propane 20 isobutane 20 n-pentane 20arrow_forwardThe lower heating value for gaseous n-octane is 44,791 kJ/kg, and its latent heat of vaporization is 300 kJ/kg. Using this information, determine the enthalpy of formation at 298 K for liquid n-octane in kJ/kmol. (Here you are to compute the enthalpy of formation from the given information, not look it up in a property table.)arrow_forwardA one-inch thick steak initially at room temperature is placed inside an oven maintained at 250 o C. Estimate how long it will take for its internal temperature to reach 70 o C. What will be the surface temperature at that time if we assume no steam generation? Include radiation as well as convection (h ~ 3 W/m²/K) in your analysis.arrow_forward
- A single effect evaporator was used to concentrate 8000 kg / hr of tomato juice from 5% total solids to 30% total solids. The juice enters the evaporator at 15 ° Celsius. The evaporator was operated by steam (80% quality) at 143.27 kPa. The vacuum in the evaporator allows the juice to boil at 80 ° Celsius. the specific heat of the Ecer material is 4.1 KJ / (kg degrees Celsius) and the concentrate product is 3.1 KJ / (kg degrees Celsius). Count it a. Steam demand rate = b. Steam economy if Condensate temperature is released at 75 ° Celsius =arrow_forwardAs part of a constant pressure calorimetry experiment for a reaction in water, you measured a temperature change from 275 K to 300 K for the water in the calorimeter. How much heat in Joules was released by the reaction if the calorimeter had 10 mL of water in it (assuming that the density of water = 1.0 g/mL)?arrow_forwardPlease include your diagram, mass balances, and full work. Thank you.arrow_forward
- 05: Acetylene gas (C₂H₂) at 25 °C is burned during a steady-flow combustion process with 30 percent excess air at 27 °C. It is observed that 75x10³ kJ of heat is being lost from the combustion chamber to the surroundings per kmol of acetylene. Assuming combustion is complete, determine the exit temperature of the product gases.arrow_forward3arrow_forwardA single effect evaporator was used to concentrate 8000 kg / hr of tomato juice from 5% total solids to 30% total solids. The juice enters the Evaporator at 20 ° C. The evaporator is operated on Steam (85% quality) at 143.27 kPa. The vacuum in the evaporator allows the juice to boil at 80 ° C. The specific heat of the dilute material is 4.1 kJ / (kg ° C) and the concentrate product is 3.1 kJ / (kg ° C). Count it (a) Steam demand rate = Answerkg / hour. b. Steam economy when condensate temperature is released at 75 ° C. = Answer(kg of water evaporated / kg of steam)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The