
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
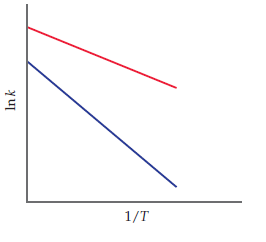
The accompanying graph shows plots of ln k versus 1/T
for two different reactions. The plots have been extrapolated
to the y-intercepts. Which reaction (red or blue) has
(a) the larger value for Ea, and (b) the larger value for the
frequency factor, A?
Transcribed Image Text:1/T
Ink
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rate information was obtained for the following reaction at 33 °C; The value of the rate constant k = 2 x 10³ at 25°C. Cr(H₂O)6³+ + SCN ---> Cr(H₂O)5NCS²+ + H₂O(1) @33 °C Initial Rate 2.0x10-¹1 2.0x10-10 9.0x10-10 2.4x10-⁹ 1.4x10-10 [Cr(H₂O)6³*] 1.0x104 1.0x10-³ 2.0x10-³ 3.0x10-³ 1.0x10 [SCN"] 0.10 0.10 0.15 0.20 0.20arrow_forwardThe elementary reactionearnin2H, 0(g ) = 2H2(g) + 02(8)millanproceeds at a certain temperature until the partial pressures of H, O, H2, and O, reach 0.0350 atm, 0.00600 atm, and = 0.00500 atm, respectively. What is the value of the equilibrium constant at this temperature?arrow_forwardConsider the reaction below A products where k = 0.0882 s-1 If the initial concentration of A is 0.377 M, how many seconds will it take for A to be 36.7% consumed? potentially useful information: In([A] / [A],) = - kt 1/[A] = kt + 1/[A], [A] = -kt +[A],arrow_forward
- 74x10+8 0.100 M, calculate the 104&the inale A + B 2C K = 1.0x103 If the observed concentrations at time t are all 0.100 M, will the reaction from that point on proceed to products or reactants? If the observed concentrations at time t are 0.400 M A, 0.500 M B and 0.0100 M C, will the reaction from that point on proceed to products or reactants? to enoiletineonoo muhdiliupe erlt etsluols M 00 s8 & A to O bns 8 A noitsineono lsitini edi 80xes.r=arrow_forwardDinitrogen tetraoxide partially decomposes according to the following equilibrium: N2Oatg) 2NO219) A 1.00L flask is charged with 0.479 moles of N204. At equilibrium at 380K, 0.176 moles of N204 remains. K. for this reaction is Write your answer to 3 Sig Figs (ie. 1.23EO)arrow_forwardConsider the quilibrium reaction between X and Y, as shown below: X=Y AG The reaction is started with 10 mmol of X; no Y is initially present. After 48 hours, analysis reveals the presence of 10 mmol of X and 0 mmol of Y. Which is the most likely explanation? = −1 - 45 kJ mol X and Y have reached equilibrium concentrations. An enzyme has shifted the equilibrium toward X. Formation of Y is kinetically slow; equilibrium has not been reached by 48 hours. Formation of Y is thermodynamically unfavorable. Two of the above explanations are reasonable.arrow_forward
- 6:32 PM Sun Feb 5 How long will it take for the concentration of A to decrease from 0.910 M to 0.483 for the reaction A → Products? (k = 0.153 M/S) Tap here or pull up for additional resources 38 1 n @ 2 W #3 F 4 R 45 Question 34 of 50 % < 6 & 7 * CO 8 1 4 7 +/- 9arrow_forwardA reaction mixture is made of initial: [PCl5] = 0.210 M, [PCl3] = 0.0900 M, [Cl2] = 0.0900M In which direction will the reaction proceed to reach eqºm? Q = ------ = _____Mx______M/_______M > ? < K = 0.042M shifts? If [PCl5]eq = 0.2065 M, find all [conc]eq in this reaction mixture. Set up an ICE table: [PCl5] [PCl3] [Cl2] Init Change Eqºm [PCl5]eq = 0.2065 M = _______ x = _______ M [PCl3]eq = ___________ = _______ M [Cl2]eq = _______ Marrow_forwardA. Consider the endothermic reaction at 25oC: FeSCN+2(aq) --><-- Fe+3(aq) + SCN-(aq) What direction will the reaction shift in response to the following stresses? (Use arrows or write "right" , "left" , or "no shift"). a. More SCN- is added. b. Solid sodium hydroxide is added. (iron (III) hydroxide is insoluble). c. The reaction container is set into ice water to cool. d. The pressure of the air around the reaction is doubled. e. Solid iron (III) chloride (FeCl3) is added. B. At 25oC, Kc for thr above reaction is 9.1 x 10-6. Determine the equilibrium concentration of Fe+3 in the solution if 0.50 mol of FeSCN+2 is dissolved to make 2.50 liters of solution.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY