
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
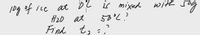
Transcribed Image Text:at D2 is mixed
with sag
1og of ice
58°2?
ber
A20 at
Find t, :."
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please solve this ACTIVITY Farrow_forwardA liquid has its vapor pressure measured at several temperatures. A graph of the ln P vs 1/T is prepared and appears below. If the equation for the line is.. y = -4699.3 k X + 2.38 What is ΔHvap for the liquid in kJ/mol? Answer should be 4 sig figs.arrow_forwardGive detailed Solution....don't give Handwritten answerarrow_forward
- Nitroglycerin is a dangerous powerful explosive that violently decomposes when it is shaken or dropped. The Swedish chemist Alfred Nobel (1833-1896) founded the Nobel Prizes with a fortune he made by inventing dynamite, a mixture of nitroglycerin and inert ingredients that was safe to handle. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of liquid nitroglycerin (C₂H5(NO3)₂) dioxygen, gaseous water and gaseous carbon dioxide. into gaseous dinitrogen, gaseous 2. Suppose 13.0 L of carbon dioxide gas are produced by this reaction, at a temperature of -11.0 °C and pressure of exactly 1 atm. Calculate the mass of nitroglycerin that must have reacted. Round your answer to 3 significant digits. ローロ X 00 0° 2 画 oloarrow_forwardPlease don't provide handwritten solution ....arrow_forwardIf 1000 L of gasoline has spilled into the street (MW=117 g/mol, density=0.7 g/mL which is true for octane) then firefighters add a lot of a material that dissolves into it that reduces the vapor pressure (liquid octane doesn’t burn, only the vapors). The firefighters put 500 kg of a material into the gasoline and reduced its vapor pressure from the pure partial pressure of 10.0 kPa down to 8.0 kPa. What is the molecular weight of the material that was added? (hint: use Raoult’s Law, which relates changes in pressure to the mol fractions.arrow_forward
- a sample of dolomitic limestone containing only CaCO3 and MgCO3 was analyzed a) when a 0.2800-gram sample of this limestone was decomposed by heating, 75.o milliliters of CO2 at 750. mm Hg and 20°C were evolved. How many grams of CO2 were produced? b) write equations for the decomposition of both carbonates described above. c) It was also determined that the initial sample contained 0.0448-gram of calcium. What percent of the limestone by mass was CaCO3? d) How many grams of the magnesium-containing product were present in the sample in a. after it had been heated?arrow_forwardGypsum is a relatively soft rock made of CaSO4. Rainwater percolates through gypsum, dissolves some of the rock, and eventually becomes saturated with Ca2+ ions and SO²- ions. A geochemist takes a sample of groundwater from a cave and finds that it contains 8.4 x 10-3 M SO42- and 5.8 x 10-3 M Ca²+. (The ratio is not 1:1 because other sulfate rock contributes some of the SO42- ions to the solution.) Use these data to determine the solubility product of CaSO4.arrow_forwardA certain solid substance that is very hard, has a high melting point, and is nonconducting unless melted is most likely to be Cuntien aloitts and starca by v ceiis, luge alvītis ar StarCa NY TLU face atoms are shared by 2 cells, and atoms in the middle of the c KCI atoms per cell NO ₂ Cr noies giving a molecular 2 at solv rmula oT IVL.X.. H₂Oarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY