
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Can you please explain for me the best way to do this problem?
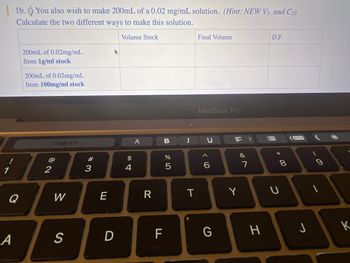
Transcribed Image Text:1b. You also wish to make 200mL of a 0.02 mg/mL solution. (Hint: NEW V₂. and C2).
Calculate the two different ways to make this solution.
Volume Stock
Q
A
200mL of 0.02mg/mL
from 1g/ml stock
200mL of 0.02mg/mL
from 100mg/ml stock
Body A
@
2
W
S
#3
E
$
D
A
R
I
B
F
65°
%
I
T
Final Volume
MacBook Pro
U
A
< 6
G
Y
&
7
H
D.F.
* 00
U
J
69
1
K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- classify the molecules of interest (i.e. para-aminophenol, acetaminophen, phenacetin) in increasing order of polarity? Rationalize these observations based on molecular interactions.arrow_forwarde) emulsifiers are pretty important compounds for daily life, externally and internally to us humans. Describes the two parts of an emulsifier molecule, and how most emulsifiers work and what they actually do.arrow_forwardI tried this and got it wrongarrow_forward
- Which of the following can complete the reaction below? O Br2/FeBr3 O NBS/(PhCO2)2 O H2SO4 in boling water O H₂, Pt, Ethanol at 130 atm. ?arrow_forward7. Which of the following would behave as a hydrogen bond acceptor, but not a hydrogen bond donor? H H:0: H I || | Н-с-с-с-н H :0: H ннн ннн Н-с-с-с—Н Н-с-с-с-о- Н-с-с-с-N-H ннн H ннн ннннarrow_forwardSelect the single best answer. Intramolecular forces of attraction are often important in holding large molecules together. For example, some proteins fold into compact shapes, held together by attractive forces between nearby functional groups. A schematic of a folded protein is shown below, with the protein backbone indicated by a blue-green ribbon, and various appendages drawn dangling from the chain. What type of intramolecular force occurs at site B? E NH, CH, -HOCH;- O=C H-N hydrogen bond CCH, CH, CH,-S S-CH, hydrogen bond -CH,CH(CHl2 CH CH, CH CHCH -CH, helical structure | disulfide bond van der Waals forces dipole-dipole interactions hydrogen bonding ion-ion interactionsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY