
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
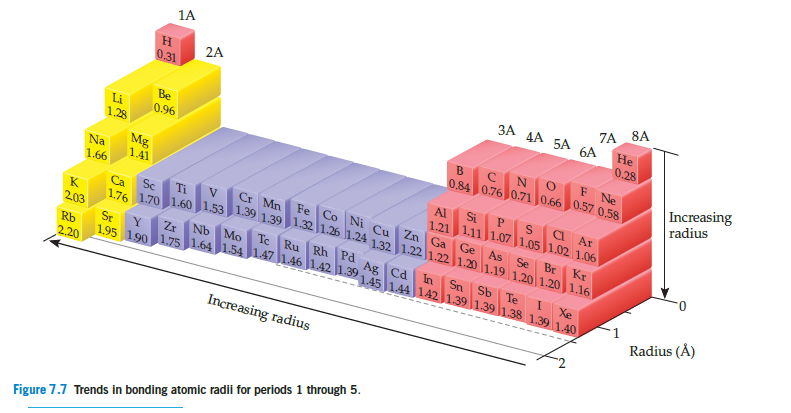
In the series of group 5A hydrides, of general formula
MH3, the measured bond distances are P¬H, 1.419 Å;
As¬H, 1.519 Å; Sb¬H, 1.707 Å. (a) Compare these values
with those estimated by use of the atomic radii in
Figure 7.7. (b) Explain the steady increase in M¬H bond
distance in this series in terms of the electron configurations
of the M atoms.
Transcribed Image Text:1A
Н
2A
0.31
8A
7A
3A 4A
БА
6A
He
0,28
Be
0.96
Li
1.28
Increasing
radius
Ne
0,84 0.76 0.71 0.66 0.57 0,58
Mg
1.41
Na
1.66
Si
P.
Al
Ar
1.21 1.11 1.07 |1.05 1.02 1.06
Kr
60 1.53 1.39 1.391.32 |l.26 1.24 1.32 1.22 1.22 1.20 1.19 1,20 1.20 1.16
Cr Mn
Fe Co Ni Cu Zn Ga
Ti
Ca
Sc
a
K
2.03
Ge As
Se
Br
Nb Mo
Tc
Ru Rh Pd
Sr
Y.
Zr
1,95 190 1.75 1.64 1.54 147 1.46 1,42 1391451.44 1 42 139 1.39 1.38 1.39 1.40
Rb
Ag Cd
In
Sn
Sb
Te
2.20
Xe
Radius (Å)
Increasing radius
Figure 7.7 Trends in bonding atomic radii for periods 1 through 5.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe the molecular shape of the following: (a) NCl2+arrow_forward7.83 In the series of group 16 hydrides, of general formula MH2, the measured bond distances are 0-H, 96 pm; S-H, 134 pm; Se-H, 146 pm. (a) Compare these values with those estimated by use of the atomic radii in Figure 7.7. (b) Explain the steady increase in M-H bond dis- tance in this series in terms of the electron configurations of the M atoms.arrow_forwardThe pentafluorides of the larger members of Group 5A(15)have been prepared, but N can have only eight electrons. A claim has been made that, at low temperatures, a compound with the empirical formula NF₅ forms. Draw a possible Lewis structure for this compound.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY