
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
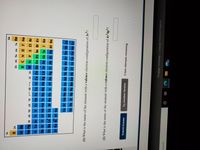
Transcribed Image Text:1A
8A
H 2A
3A 4A 5A 6A 7A He
Li Be
Na Mg 3B 4B 5B 6B 7B 8B- 1B 2B AI| Si P
K Ca Sc Ti VCr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te IXe
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn
米
**
Fr Ra Ac Rf Ha
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Th Pa UNp Pu Am Cm Bk Cf Es Fm Md No Lr
(1) What is the name of the element with a valence electron configuration of 3s'?
(2) What is the name of the element with a valence electron configuration of 4s24p1?
Submit Answer
Try Another Version
5 item attempts remaining
Cengage Learning | Cengage Technical Support
search
近 0
20,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1A H2A Li Be 4A 5A 6A 7A He CNOF Ne Cl Ar Na Mg 3B 48 58 68 7888 Si P S K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Pd Ag Cd In Sn Sb Te I Xe Rb Sr Y Zr Nb Mo Tc Ru Rh Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Rc Rf Ha C Submit Answer 3A 1B 2B Al 8A Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr A monatomic ion with a charge of +2 has an electronic configuration of 1s²2s²2p63s23p64s²3d¹04p6. This ion is a(n) It has the same electron configuration as the noble gas The symbol for the ion is: Retry Entire Group eded for this question. 8 more group attempts remainingarrow_forward1A H2A Li Be Na Mg 3B 4B 5B 88 K Ca Sc Ti V Cr Mn Fe Co Ni 6B 7B **** This ion is a(n) 3A 4A 5A 6A 7A He BCN OF Ne Si P S Cl Ar 1B 2B Al 8A Cu Zn Ga Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha with a charge of Ge As Se Br Kr Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Write the complete electron configuration for the common monatomic ion formed by the element sulfur, S .arrow_forward1A 8A H 2A 3A 4A 5A 6A 7A He Li Be BC NO FNe Na Mg зв 4в 5B 6B 7B - 8B - 1B 28 A1 Si P S CI Ar K Ca Sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn ** Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr (1) The element with an electron configuration of 1s²2s²2p°3s 64s²3d6 is in group 8 and period 4 (2) The element with an electron configuration of 1s²2s²2p°3s' is in group and periodarrow_forward
- 1A 8A H 2A 3A 4A SA 6A 7A He Li Be BCNOFNe 1B 2B AISi P SCI Ar Na Mg 3B 4B 58 6B 7B 88 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Zr Nb Mo Tc Ru Rh Pd Rg Cd In Sn Sb Te Xe CS Ba La Hr Ta w Re Os Ir Pt Au Hg T Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Ma No Lr (1) What is the valence electron configuration for the arsenic atom? (2) What is the valence electron configuration for the carbon atom?arrow_forward1A H2A 3A 4A 5A 6A Li Be Na Mg 38 4B 5B 6B 7B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Cs Ba La Hf Ta Fr Ra Ac Rf Ha 8B BA B C N 0 Cl Ar 1B 2B Al Si P S Ge As Se Br Kr Sn Sb Te I Xe We Os Ir Pt Au Hg Tl Pb Bi Po At Rn 7A He F Ne Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Write the complete electron configuration for the manganese(II) ion. Using NOBLE GAS notation write the electron configuration for the cobalt(II) ion.arrow_forward1A 8A H 2A 3A 4A 5A 6A 7A He Li Be CNO F Ne Na Mg 3в 4B 5B 6B 78 — 88 — 1B 2B A1 SIPS CI Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn ** Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Write the complete electron configuration for the zinc ion. Using NOBLE GAS notation write the electron configuration for the copper(II) ion.arrow_forward
- 1A 8A H 2A 3A 4A SA 6A 7A He CNOF Ne Li Be В B 2B ASi PS CI Ar Na Mg 3B 4B 5B 6B 7B г 68 K Ca Sc TiV Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Xe Rb Sr Y Zr ND MO Tc Ru Rh Pd Ag ca In Sn Sb Te Cs Ba La Hf Ta Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa UNp Pu Am Cm Bk Cf Es Md No Lr Considering only ions with charges of+1, +2, -1 and -2, or neutral atoms, give the symbols for 4 species that are isoelectronic with the chloride ion, Clarrow_forward1A 8A H 2A 3A 4A 5A 6A 7A He Li Be BC NO F Ne Na Mg 38 4B 5B 6B 7B -88 - 1B 28 AI si P S CI Ar K Ca sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn ** Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr (1) What is the element with an electron configuration of 1s²2s²2p°3s²3p°4s²3a$? (2) What is the element with an electron configuration of 1s2s²2p°3s²3p°4s?arrow_forward1A 8A H 2A 3A 4A SA 6A 7A He Li Be B C NO F Ne Na Mg 38 4B 5B 68 7B r 8B - 1B 28 AI Si PS CI Ar K Ca Sc Ti v Cr Mn Fe Co Ni Cu zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Rg Cd In Sn Sb Te I Xe Cs Ba La Hf Ta w Re Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Cf Es Fm Md No Lr Using only the periodic table arrange the following elements in order of increasing ionization energy: silicon, aluminum, sodium, phosphorus Lowest Highest Please answer this question according to the general rules you have learned regarding periodic trends. DO NOT base your answer on tabulated values since exceptions may occur. Submit Answer Retry Entire Group No more group attempts remainarrow_forward
- 1A H 2A Li Be 8A 3A 4A 5A 6A 7A He BCN OF Ne S Cl Ar 1B 2B Al Si P Cu Zn Ga Pd Ag Cd In Pt Au Hg Tl Na Mg 3B 4B 5B 6B 7B8B K Ca Sc Ti V Cr Mn Fe Co Ni Rb Sr Y Zr Nb Mo Tc Ru Rh Cs Ba La Hf Ta W Re Os Ir Fr Ra Ac Rf Ha ** Ge As Se Br Kr Sn Sb Te I Xe Pb Bi Po At Rn Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr (1) The element with an electron configuration of 1s²2s²2p63s²3p² is in group (2) The element with an electron configuration of 1s²2s²2p63s²3p³ is in group and period and periodarrow_forwardLi Be N. OF Ne Na Mg 3B 4B 5B 6B 7B 8B- 1B 2B A1 Si PS CI Ar K Ca Sc Ti V Cr Mn Fe Co NI Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo To Ru Rh Pd Ag Cd In Sn Sb Te Xe Cs Ba La H Ta W Re Os Ir Pt Ru Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cr Es Fm Md No Lr (1) What is the complete ground state electron configuration for the carbon atom? 1s2s22p² (2) What is the complete ground state electron configuration for the iron atom? Submit Answerarrow_forwardSmallest Largest 1A H 2A neon Li Be Na Mg 38 4B 5B 6B 7B8B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Rb Sr Y Zr Nb Mo Tc Ru Rh Cs Ba La Hf Ta W Re Os Ir _** Fr Ra Ac Rf Ha 3A 4A 5A 6A 7A He BC N O F Ne 1B 2B Al Si P S Cl Ar Ge As Se Br Kr Pd Ag Cd In Using only the periodic table arrange the following elements in order of increasing atomic radius: neon, krypton, helium, argon. argon 8A Sn Sb Te I Xe Pt Au Hg Tl Pb Bi Po At Rn Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Please answer this question according to the general rules you have learned regarding periodic trends. DO NOT base your answer on tabulated values since exceptions may occur. Drag and drop your selection from the following list to complete the answer: helium krypton Previous Nexarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY