
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:AutoSave
File Home Insert Draw Design
X
Paste
Clipboard
Page 4 of 9
Off
Times New Roman 11
BIU ab x₂x²A 2. A
CURE What's in Your Water - Introduction and Prelab Ass
Layout References Mailings Review View
A A Aa A · E ·5·|==|
= = = -
Font
61°F
Cloudy
Next, look at Graph 2.
Based on this graph, if you make
less than $39,524 per year, what is
the average number of hazardous
waste sites within one square mile
of you?
2295 words
What about if you make more than
$65,876 per year?
F
What does this tell you about
where hazardous waste sites might
be located, and who might be
affected by them?
English (United States)
Text Predictions: On
O Search
Paragraph
Accessibility: Investigate
Transcribed Image Text:s in Your Water-In...
Summary:
Mean Number of Sites per
Square Mile
Summary:
2
https://www.learningforjustice.....
F2
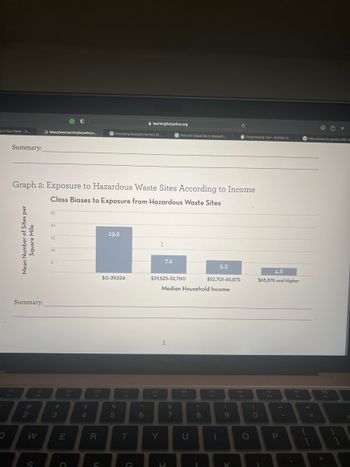
Graph 2: Exposure to Hazardous Waste Sites According to Income
Class Biases to Exposure from Hazardous Waste Sites
25
20
15
10
5
3
80
E
D
$
4
F4
R
LL
U Protecting Georgia's Surface W...
19.2
$0-39,524
%
5
FS
T
learningforjustice.org
co
6
I
Y
7.2
5.3
$52,701-65,875
Median Household Income
$39,525-52,700
1
L
Pollution Disparities in Massach...
&
N
7
44
FT
U
* CO
8
1
DII
FO
-
9
K
Paraphrasing Tool - QuillBot Al
DD
F9
O
4.6
$65,876 and Higher
0
1
4
F10
P
;
F11
Ⓒ +
https://www.nrc.gov/docs/ML13
11
11
d
Expert Solution
arrow_forward
Step 1: Graphical Interpretation
If I make less than $39524, then it corresponds to value 19.2 on the y-axis which means that the average number of hazardous waste sites within one square mile for me will be 19.2
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate Toms new ratio. Are his values within a normal range?arrow_forward17.28 Write the base sequence in a complementary DNA segment if each original segment has the following base sequence: a. TTTTTT b. c. ATGG CA d. ССССССccc ATATGCGCTAAA 88.femsidon DNAarrow_forward6. 5. 3. 4. 1. 2. 73 Discussion questions In general, how can the organic and aqueous layers be distinguished in a separating funnel? Jasin 199x3 What can cause pressure build-up in a separating funnel during an extraction? Some students find that the combined weight of their crystals is more than 2g. How might this occur? Students usually recover less than 2g of crystals. At which stage(s) is loss of material likely? Write a balanced net ionic equation for the chemical reaction that occurs in step 2. Use RCOOH to represent your unknown carboxylic acid. Write balanced net ionic equations for the two chemical reactions that occur in step 4. Again, use RCOOH to represent your unknown carboxylic acid.arrow_forward
- 3. 4. 6. 8 6. 10 12 13 14 The acid dissociation constant K of alloxanic acid (HC,H,N,O,) is 2.24 x 10. Calculate the pH of a 3.0M solution of alloxanic acid. Round your answer to 1 decimal place. dla pH = Submit Assignment Continue MacBook Air FI F10 R00arrow_forwardProblem 3. (6.27, From Chang's Book). The calcium ion binds to a certain protein to form a 1:1 complex. The following data were obtained in an experiment: Total Ca2+ (uM) 60 120 180 240 | 480 Ca2+ bound to Protein (uM) 31.2 51.2 63.4 70.8 | 83.4 Determine graphically the dissociation constant of the Ca2+ - protein complex. The protein concentration was kept at 96 uM for each run. (1 pM = 1 x 10- 6 M.)arrow_forward2.2.1 Gene T X Inbox (9,414 × M Verify your e X My Question x Things I Can x Course Hom x + USC A openvellum.ecollege.com/course.html?courseld=16196689&HepID=aeb74d6324e98a415a0c4c5c32b9c2e6#10001 I Review | Constants | Periodic Table MISSED THIS? Read Section My Courses 16.8 (Pages 701 - 710) ; Watch KCV 16.8, IWE 16.9. Part A Course Home Consider the reaction What will be the equilibrium concentration of CO? Syllabus CO (g) + H2O (g) CO2 (g) + H2 (g) Express the concentration in molarity to two significant figures. K. = 102 at 500 K Scores A reaction mixture initially contains 0.115 M CÓ and 0.115 M H2O. ? Pearson eText [CO] = M Study Area Document Sharing Submit Request Answer User Settings Part B Complete previous part(s) Course Tools > Part C Complete previous part(s) Part D Complete previous part(s) P Pearson Copyright © 2020 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy Permissions Contact Usarrow_forward
- Give correct answerarrow_forwardLIFE SCIENCES/GRADE 10 MDE/AUGUST 2024 FET SECTION B QUESTION 2 2.1 The diagram below shows part of the nitrogen cycle and the water cycle. B A 2.1.1 Identify the process at A where nitrogen in the atmosphere is converted into a usable form for plants. 2.1.2 in which form is nitrogen absorbed by plants out of the ground at B? (1) (1) 2.1.3 Which part of the water cycle is represented by C. (1) 2.1.4 Runoff water from the farm into the river at D can cause excess nutrients / fertilizers to flow into the water. What is this phenomenon called? (1) 2.1.5 Explain how the process mentioned in QUESTION 2.1.4 above might cause organisms to die in the river. ࿉C (3) (7) COPY RIGHT RESERVED PLEASE TURN OVERarrow_forward1. Identify the amino acids with the following components in their R group: 1.1. Hydroxyl group 1.2. A sulfur atom 1.3. A second chiral carbon 1.4. An amide group 1.5. An amino group 1.6. A branched side chain 2. Compare and contrast the following pairs: 2.1. Cysteine & Cystine 2.2. Reduced glutathione & Oxidized glutathione 2.3. Lysine & Hydroxylysine 2.4. D-amino acid & L-amino acid .Draw the titration curves of an acidic amino acid, a basic amino acid, and a neutral amino acid ased on their R group. Explain also how titration of amino acids is performed.arrow_forward
- Question 4 What is the isoelectric point of the tripeptide gly-ala-cys? pka values are as follows: Gly: 2.3, 9.6 Ala: 2.4, 9.7 Cysteine: 1.7, 8.3 (R group), 10.8 9.0 5.7 10.3 6.6 5.0arrow_forward4) You are stranded and have two plastic jugs, you decide to burn one of the jugs to to send smoke signals and to keep yourself warm, and you decide to save the other jug to carry and store water. One jug is made out of polyvinyl chloride (PVC) and one is made out of polypropylene (PP). Which jug do you choose to burn and why? Combustion of PVC Combustion of PP AHFPVC = 29.6 kJ/mole C₂H3Cl(g) + O₂(g) → CO₂(g) + H₂O(g) + HCl(g) C3H6(g) + O2(g) → CO₂(g) + H₂O(g) AHFPP = 20.6 kJ/molearrow_forward2.2.1 Gene T x m Inbox (9,414 × M Verify your e X My Question x Things I Can x Course Hom x + USC A openvellum.ecollege.com/course.html?courseld=16196689&HepID=aeb74d6324e98a415a0c4c5c32b9c2e6#10001 I Review | Constants | Periodic Table MISSED THIS? Read Section 16.6 (Pages 696 - 699) ; Watch IWE 16.5 . Part A Consider the following reaction: Fe3+ (aq) + SCN (aq) = FESCN²+ (aq) A solution is made containing an initial Fe+ of 1.2x10-3 M and an initial [SCN ] of 8.1×10-4 M . At equilibrium, [FESCN²+] = 1.8×10-4 M . Calculate the value of the equilibrium constant (Kc). Express your answer using two significant figures. View Available Hint(s) Πνα ΑΣφ ? K = Submit Previous Answers Request Answer * Incorrect; Try Again; 2 attempts remaining Provide Feedback Next :arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY