
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Give typed answer not written
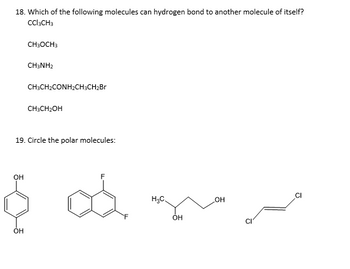
Transcribed Image Text:18. Which of the following molecules can hydrogen bond to another molecule of itself?
CCl₂CH3
CH3OCH3
CHINH2
CH3CH₂CONH₂CH3CH₂Br
CH3CH₂OH
19. Circle the polar molecules:
OH
$ ad
OH
H₂C.
OH
OH
J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name N-chloromethylamine methanimine compound bromomethane formula or Lewis structure H | H-C H H | - CI: H | H-C=N-H CH₂ Br Between molecules of the compound? O O ΟΟ yes no yes no yes hydrogen-bonding force no Between molecules of the compound and molecules of water? O O ОО yes no yes no yes no X 5arrow_forwardcompound hydrogen-bonding force Between Between molecules of formula or Lewis molecules of the the compound and molecules of water? name structure compound? H H O yes yes ethane Н — с —с — н no no H H H H yes çi: yes N-chloromethylamine Н—с —N no no H yes yes iodomethane CH,I no no O O O O :ö:arrow_forwardFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name hydrogen lodide methanimine compound dimethyl ether formula or Lewis structure H HIC NIH H HI H H-C-6-C-H HICIH Between molecules of the compound? O yes O no O yes O no hydrogen-bonding force. O yes O no Between molecules of the compound and molecules of water? Oyes O no O yes O no O yes O no ola Earrow_forward
- For each compound in the table below, decide whether there would be any hydrogen-bondir of the compound and molecules of water. compound hydrogen-bonding force Between Between molecules of formula or Lewis molecules of the compound? the compound and molecules of water? name structure H :O: yes yes acetic acid Н-с — с — о — н no no H. yes yes formyl chloride CHOCI no no H yes yes methanol H - C-0- H no no H.arrow_forwardfrom lowest to highest, 4 being the lowestarrow_forwardWhich molecules can hydrogen bond ? CH4 HF H2 CH3OHarrow_forward
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. chemical symbol, substance chemical formula boiling point or Lewis structure Ar (Choose one) A В Cr (Choose one) v H H. H. H - C - C - ) - C H (Choose one) | H H H. H H - C -H D :N - O - H (Choose one) ♥ Н—С — Н ? :0 : :arrow_forward17 -OH II II он он IV Which compound would have the lowest boiling point? S O II IVarrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. chemical symbol, chemical formula or Lewis structure substance boiling point A Cr (Choose one) H Н— С —Н :N-O –H (Choose one) v Н — С — Н H Ar (Choose one) v H H Н— С — С — О — С — Н (Choose one) v H H Harrow_forward
- According to this figure: HO NH2 HN NH2 NH2 NH3 HO HO What is the type of interaction labeled A? Oal lonic bondarrow_forwardFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name methanimine methylamine ammonial compound formula or Lewis structure H H-C=N-H H H 1 C 1 H H T N-H NH₂ Between molecules of the compound? yes O no Oo yes O no yes hydrogen-bonding force O no Between molecules of the compound and molecules of water? O yes O no O yes O no O yes O noarrow_forwardFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name methanol difluoromethane ammonia compound formula or Lewis structure H I H-C-O-H I H CH₂F₂ NH₂ Between molecules of the compound? O yes O no O yes O no hydrogen-bonding force O yes O no Between molecules of the compound and molecules of water? O yes O no O yes O no O yes O no X Sarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning